JOURNAL 2546
Journal of Chemical Metrology
Year: 2022 Issue: 2 July-December
p.90 - 101
Viewed 752 times.
GRAPHICAL ABSTRACT
ABSTRACT
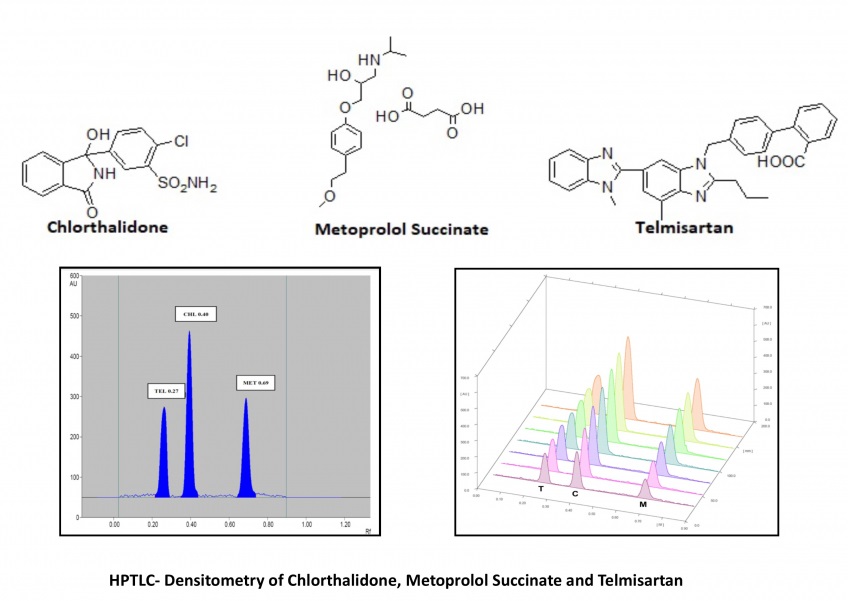
The present study shows the compilation of the results obtained for a very simple, fast and precise high-performance thin-layer chromatography (HPTLC) - densitometric determination of chlorthalidone (CHL), metoprolol succinate (MET) and telmisartan (TEL) in bulk drugs as well as the commercially available formulation. The chromatographic separation of samples was performed on Silica Gel 60 F254 aluminum sheets using Toluene: Methanol: Ethyl acetate: Tri-ethylamine as the mobile phase in the volume ratio 4:0.8:1: 1.2. The densitometric scanning was performed at 225 nm wavelength using CAMAG TLC Scanner- IV. The mentioned chromatographic system showed the compact band and symmetrical peaks of CHL, MET and TEL with 0.40 (±0.2), 0.69 (±0.2) and 0.27 (±0.2) retardation factor (Rf) respectively. The reported method is linear in the concentration range of 500-2000 ng/band, 1000-4000 ng/band and 1600-6400 ng/band while the recovery was found in the range of 98.94-99.62%, 98.26-98.41% and 99.86-100.28% for CHL, MET and TEL respectively. The method assayed the marketed formulation with 99.89 (±0.91) for CHL, 98.92 (±1.07) for MET and 100.12 (±0.65) for TEL concerning the label claim. All the results suggested the agreement of the developed method to the ICH Q2(R1) guidelines and its applicability for day-to-day analysis of these drugs in combined pharmaceutical formulations.
KEYWORDS- Analytical method
- chlorthalidone
- densitometry
- HPTLC
- metoprolol succinate
- telmisartan