JOURNAL 2596
Journal of Chemical Metrology
Year: 2022 Issue: 2 July-December
p.102 - 110
Viewed 750 times.
GRAPHICAL ABSTRACT
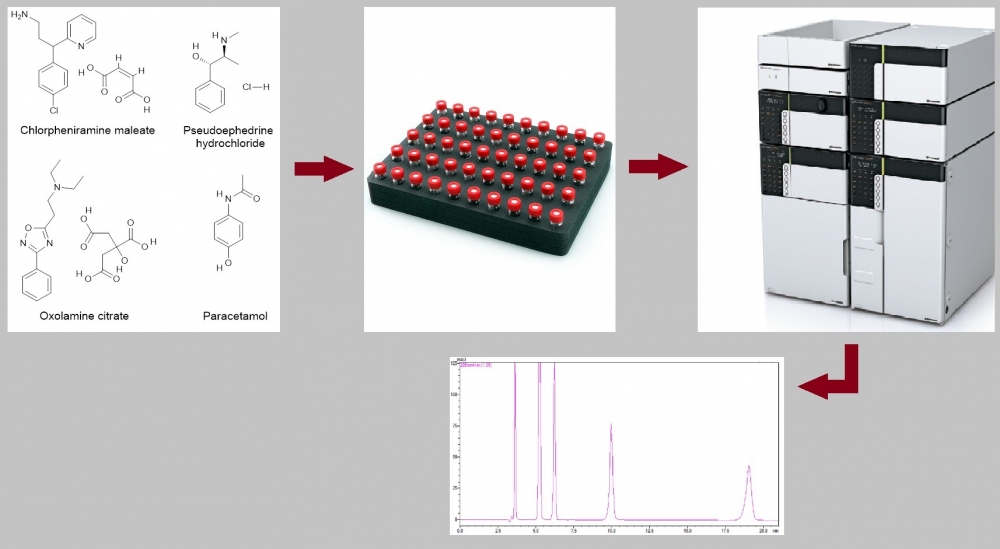
ABSTRACT
A simple and sensitive method has been developed for the simultaneous determination of chlorpheniramine maleate, pseudoephedrine hydrochloride, oxolamine citrate, and paracetamol by HPLC-PDA in pharmaceutical dosage forms. The separation of the analytes was achieved on an ACE 5 C8, 250 × 4.6 mm, 5 μm column using isocratic elution with a mobile phase containing methanol and 0.1 M phosphoric acid aqueous solution (15:85, v:v) at a flow rate of 1 mL/min. The total run time is 21 min. Chlorpheniramine maleate, pseudoephedrine hydrochloride, oxolamine citrate, and paracetamol were detected at wavelengths (retention times) of 264 nm (9.96 min), 205 nm (6.22 min), 239 nm (19.04 min) and 244 nm (5.22 min), respectively. The injection volume was 10 μL. The assay was in for chlorpheniramine maleate, pseudoephedrine hydrochloride, oxolamine citrate and paracetamol the concentration ranges 32–48 µg/mL, 9.6–14.4 µg/mL, 32–48 µg/mL, 104–156 µg/mL, respectively. LOQs (µg/mL) and LODs (µg/mL) 1.38 and 0.46 for chlorpheniramine maleate, 0.05 and 0.02 for pseudoephedrine hydrochloride, 0.76 and 0.25 for oxolamine citrate, 0.21 and 0.07 for paracetamol, respectively. Recoveries of the analytes were between 98% and 102% with intra-and inter-day precisions (as relative standard deviation) of ≤2%. In addition, expanded uncertainty values were less than 2 for all analytes. Method validation was carried out according to ICH guideline Q2 (R1). The analytical method validated was successfully applied to pharmaceutical dosage forms
KEYWORDS- Chlorpheniramine maleate
- pseudoephedrine hydrochloride
- oxolamine citrate
- paracetamol
- pharmaceutical dosage forms
- HPLC-PDA