JOURNAL 1407
Organic Communications
Year: 2019 Issue: 4 October-December
p.176 - 187
Viewed 3685 times.
GRAPHICAL ABSTRACT
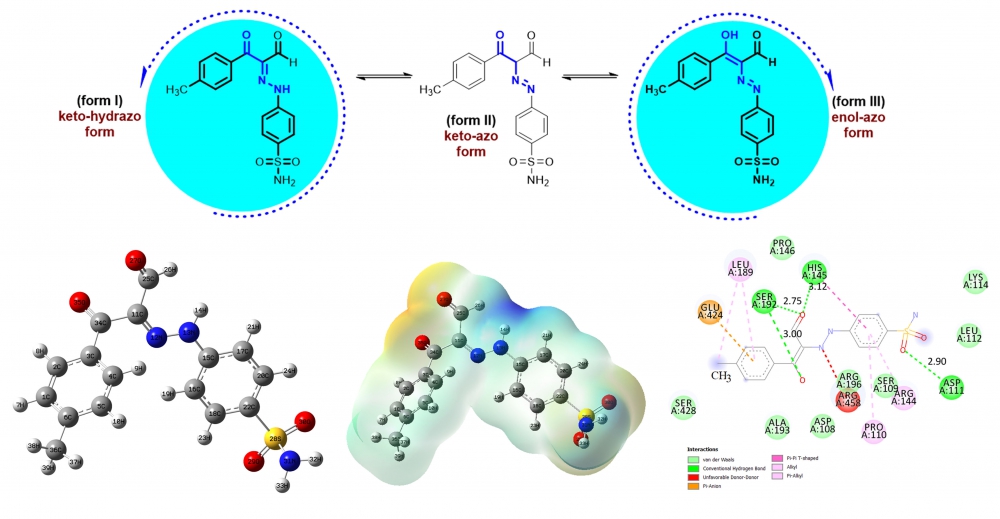
ABSTRACT
The newly synthesized six tautomeric aldehydes were characterized by 1H NMR and 13C NMR spectra. As a result of experimental characterization studies of target products, two tautomer structures named enol-azo and keto-hydrazo were observed. To support other experimental quantum chemical computations were utilized by Density Functional Theory using B3LYP functional and 6-311++G (d,p) basis set. All theoretical calculations were performed only for molecules M1 and M4 after each tautomer was optimized. The form III tautomers with lower energy of compounds M1 and M4 were determined as preferential structures in the ground state. From the MEP analysis, it is evident that the negative charge covers the C=O group and positive region is over the hydrogen groups. Finally, molecular docking calculations between 4F5S receptor and six ligand interactions were carried out by using AutoDock Vina program, and results were evaluated in detail.
KEYWORDS- Keto-hydrazo tautomer
- enol-azo tautomer
- sulfonamide
- DFT
- molecular docking
- GIAO