JOURNAL 3501
Organic Communications
Year: 2025 Issue: 2 April-June
p.83 - 99
Viewed 1306 times.
GRAPHICAL ABSTRACT
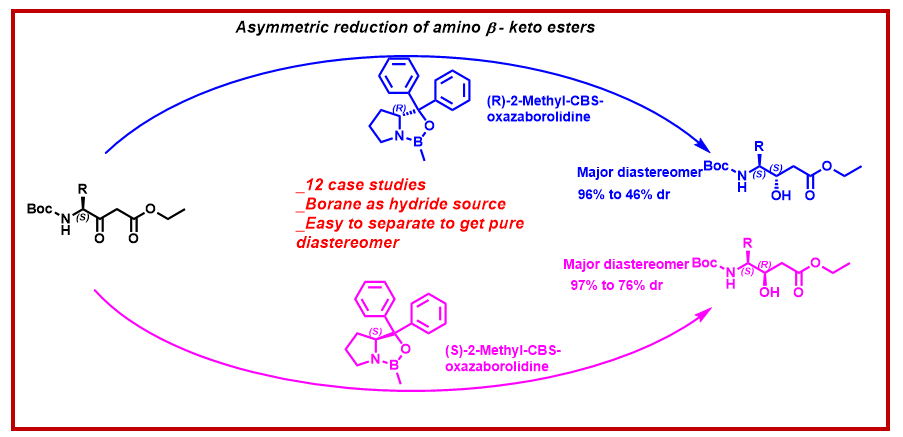
ABSTRACT
An efficient diastereoselective synthesis of Boc-protected 4-amino 3-hydroxy esters starting from natural amino acids is described. The key synthetic strategy involves diastereoselective reduction of α-L-amino-β-keto esters in the presence of enantiopure R and S-2-Methyl-CBS-oxazaborolidine catalysts using borane as hydride source. The product diastereoselectivity depends upon the use of (R) or (S) enantiomer of 2-Methyl-CBS-oxazaborolidine. A reasonable mechanism is included which explains the diastereoselectivity of the reactions with the use of different enantiomers of 2-Methyl-CBS-oxazaborolidine. Furthermore, the resulting diastereomeric mixture of the reduced products can be separated by column chromatography to gain access to the single pure diastereomers.
KEYWORDS- L-amino-β-keto esters
- statine;
- (R)-2-Methyl-CBS-oxazaborolidine
- (S)-2-Methyl-CBS-oxazaborolidine
- borane reduction