JOURNAL 3413
Organic Communications
Available Online: April 29,2025
p.1 - 23
http://doi.org/10.25135/acg.oc.187.2501.3413 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 379 times.
-
A. Hanumatha Rao

-
Ch. Subramanyam

-
K. Venkata Ramana

-
C. Gladis Raja Malar

-
G.R. Satyanarayana

-
V. Madhava Rao

GRAPHICAL ABSTRACT
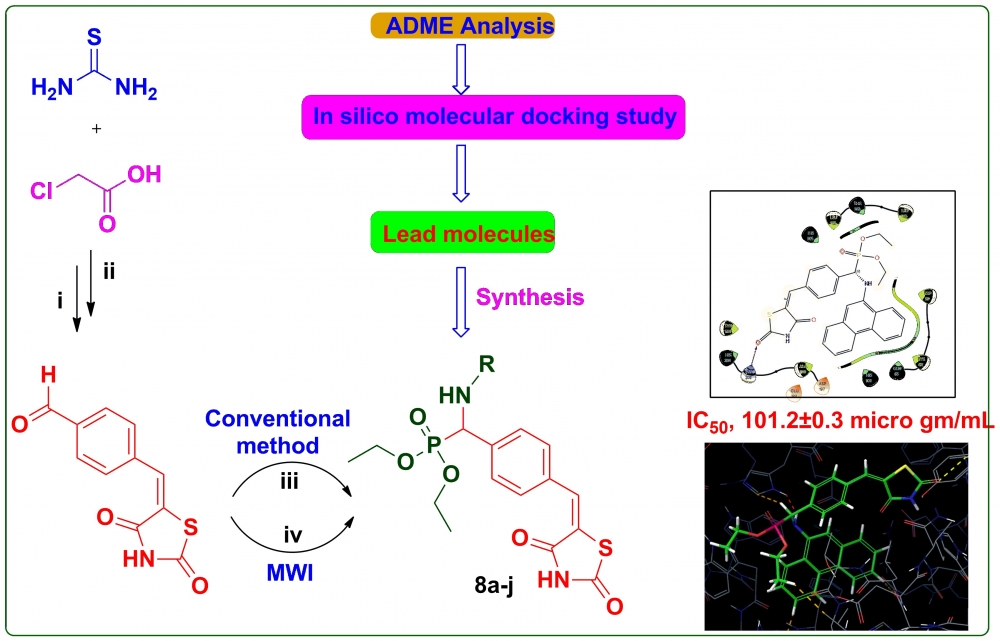
ABSTRACT
A more efficient and environmentally friendly approach has been developed for synthesizing α-aminophosphonates through the Kabachnik-Fields (K-F), catalyzed by nano-ZnO in a solvent-free environment, utilizing microwave irradiation. Before synthesis, each compound underwent in silico ADMET analysis and molecular docking to assess drug-like characteristics and their potential to inhibit α-amylase. The structure of the newly synthesized compounds was validated using spectroscopic analysis, and their in vitro inhibitory effects on α-amylase and α-glucosidase were assessed. Among the compounds 8g (IC50, 99.2±0.5 μg/mL), 8i (IC50, 101.2±0.3 μg/mL), 8e (IC50, 102.1±0.4 μg/mL) and 8j (IC50, 103.4±0.4 μg/mL) containing anthracen-9-ylamino, phenanthren-9-ylamino, 2-oxo-2H-chromen-6-yl)amino and 2-benzoylphenyl)amino moieties respectively, exhibited the highest inhibitory activity on α-amylase, superior to the reference compound acarbose (IC50, 104.5±0.1 μg/mL). The remaining compounds demonstrated moderate to good enzyme inhibition. 8c bearing with 4-fluorophenyl substituent (IC50, 88.4±0.7 μg/mL), 8e (IC50, 90.0±0.4 μg/mL) bearing with 2-oxo-2H-chromen-6-yl substituent and 8d (IC50, 91.1±0.9 μg/mL) bearing with 2-methylbenzo[d]oxazol-5-yl moiety have shown the highest inhibitory activity on α-glucosidase than the reference drug, Acarbose ((IC50, 93.1±0.8 μg/mL). The remaining compounds exhibited moderate to good inhibition on the enzyme with IC50 ranging 94.5±0.9 to 141.4±0.7 μg/mL.
KEYWORDS
- Kabachnik-Fields (K-F) reaction
- nano-ZnO,
- microwave irradiation
- molecular docking
- α-amylase
- α-glucosidase