JOURNAL 3618
Organic Communications
Available Online: October 20,2025
p.1 - 10
http://doi.org/10.25135/acg.oc.200.25.08.3618 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 129 times.
GRAPHICAL ABSTRACT
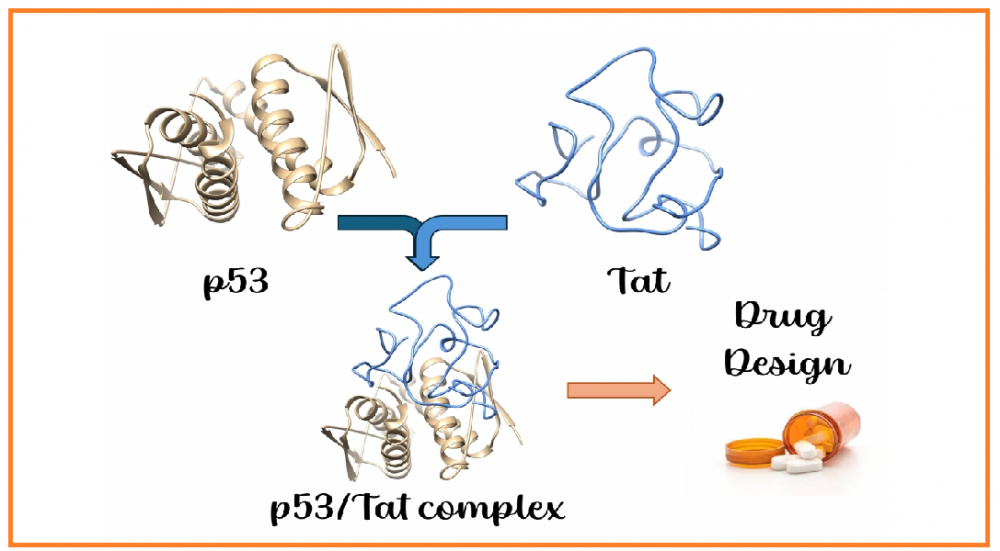
ABSTRACT
HIV-1 Tat (transactivator of transcription) protein is the main arsenal of HIV, playing numerous roles during viral infection. This protein is intrinsically disordered, lacking well-defined secondary structures. Such structural plasticity allows HIV-1 Tat to interact with a wide range of proteins and biological molecules, ultimately leading to immune system collapse or severe tissue damage. Proteomic studies have previously revealed that p53, often referred to as the “guardian of the genome,” interacts with Tat through its tetramerization domain. Since p53 plays a pivotal role in determining cell fate, its interaction with Tat is of broad interest in the pathogenesis of HIV infection. Therefore, we investigated the complex formation between Tat and the tetramerization domain of p53 using molecular docking and molecular dynamics simulations. We believe that the results presented in this manuscript provide valuable insights for the development of novel therapeutic agents targeting the p53/Tat interaction.
KEYWORDS- HIV-1/2
- Tat protein
- p53
- molecular docking
- MD simulations
- protein-protein interaction