JOURNAL 2396
Records of Natural Products
Year: 2023 Issue: 2-March-April
p.312 - 317
Viewed 3964 times.
-
Hoang-Dung Nguyen

-
Huy Truong Nguyen

-
Thi-Hoai-Thu Nguyen

-
Jirapast Sichaem

-
Huu-Hung Nguyen

-
Ngoc-Hong Nguyen

-
Thuc-Huy Duong

GRAPHICAL ABSTRACT
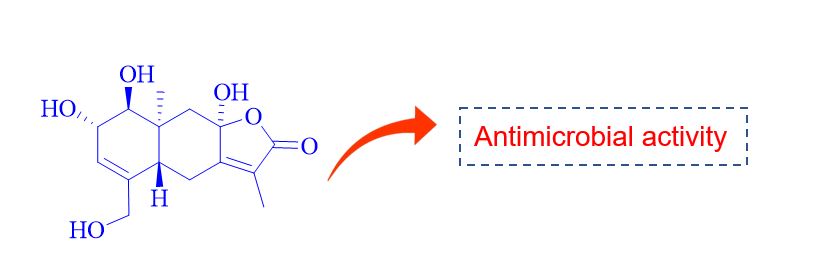
ABSTRACT
Phytochemical investigation of Lindera myrrha roots growing in Vietnam afforded a new eudesmane sesquiterpenoid, myrrhalindenane C (1), along with seven known compounds, rel-5-(3S,8S-dihydroxy-1R,5S-dimethyl-7-oxa-6-oxobicyclo[3,2,1]-oct-8-yl)-3-methyl-2Z,4E-pentadienoic acid (2), 1-O-(4-hydroxy-2,6-dimethoxyphenoxy)-6-O-[rel-5-(3S,8S-dihydroxy-1R,5S-dimethyl-7-oxa-6-oxobicyclo[3,2,1]-oct-8-yl)-3-methyl-2Z,4E-pentadienoyl]-β-D-glucopyranose (3), curcumin (4), demethoxycurcumin (5), bisdemethoxycurcumin (6), (1E,6E)-1,7-bis(4-methoxyphenyl)-1,6-heptadiene-3,5-dione (7), and 2',4',4,2''-tetrahydroxy-3'-[3''-methylbut-3''-enyl]chalcone (8). The structure was determined by analysis of their MS and NMR data as well as by comparison with literature values. All compounds were evaluated for antimicrobial activity against antibiotic-resistant, pathogenic bacteria Enterococcus faecium, Staphylococcus aureus, and Acinetobacter baumannii.
KEYWORDS- Lauraceae
- Lindera myrrha
- myrrhalindenane C
- eudesmane
- antimicrobial activity