JOURNAL 2756
Records of Natural Products
Year: 2023 Issue: 5 September-October
p.845 - 859
Viewed 2976 times.
GRAPHICAL ABSTRACT
ABSTRACT
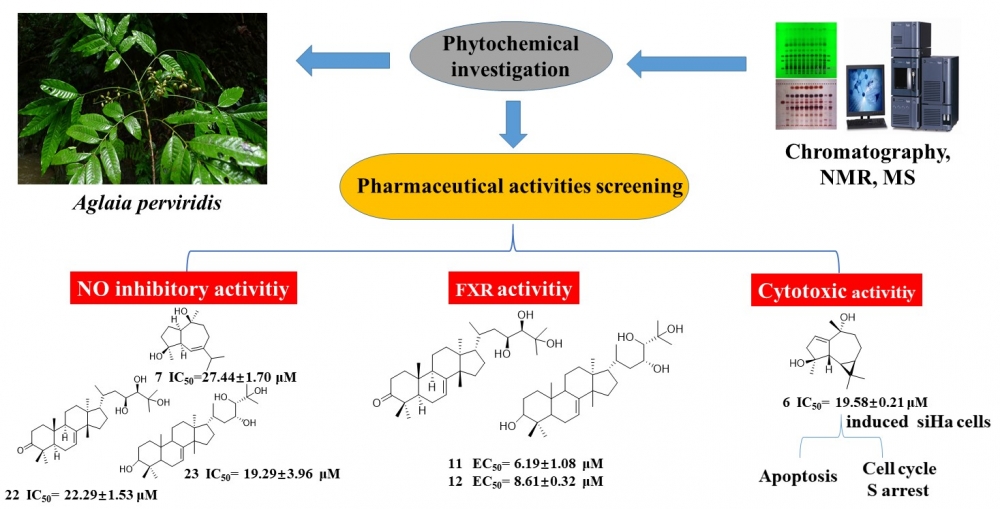
To search for novel and bioactive agents from the genus Aglaia, phytochemical investigation on the leaves and twigs of A. perviridis was performed, leading to the isolation of twenty-six compounds. Their structure was determined mainly by the NMR, ESI-MS spectra data, and by comparison with previously described compounds. Compounds 6–8, 11–13, 16, 18–20, and 22–26 were firstly reported from Aglaia perviridis. Besides, the NO inhibitory, Farnesoid X receptor, and cytotoxic activities of selected compounds were firstly evaluated. 1αH,5αH-guaia-6-ene-4β,10β-diol (7), 2'-hydroxy-4,4',6'-trimethoxychalcone (22), and 3-hydroxy-5,7,4'-trimethoxyflavone (23) showed moderate nitric oxide inhibitory activity with IC50 values of 27.44±1.70, 22.29±1.53, and 19.29±3.96 µM, respectively; piscidinol A (11) and hispidol B (12) firstly showed moderate agonistic activities on Farnesoid X receptor with the EC50 values of 6.19±1.08 and 8.61±0.32 µM, respectively; Among these compounds, lochmolin F (6) exhibited the strongest cytotoxic effect on SiHa cells with the IC50 value of 19.58±0.21 μM. Further flow cytometry and western blot analysis demonstrated that lochmolin F (6) could induce cell apoptosis and S cell cycle arrest in the SiHa cells. These results first emphasized the potential of lochmolin F (6) as lead compound for developing anti-cancer drugs.
KEYWORDS- Aglaia perviridis
- phytochemical composition
- anti-inflammatory
- FXR
- cytotoxic activities