JOURNAL 3711
Journal of Chemical Metrology
Year: 2025 Issue: 2 July-December
p.223 - 235
Viewed 246 times.
GRAPHICAL ABSTRACT
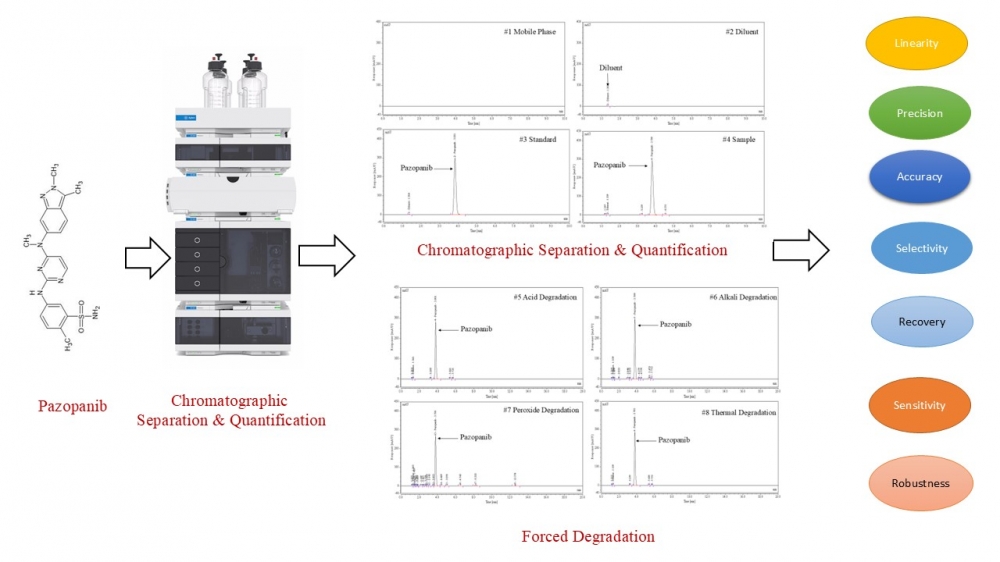
ABSTRACT
This study reports the development and validation of a novel, rapid, and stability-indicating reversed-phase high-performance liquid chromatography (RP-HPLC) method for the quantification of pazopanib in tablet formulations. Chromatographic separation was achieved on an Inertsil ODS-3V column (150 mm × 4.6 mm, 5 µm) using an isocratic mobile phase of 0.1% trifluoroacetic acid and acetonitrile (70:30, v/v) at a flow rate of 1.0 mL/min with UV detection at 268 nm. The method demonstrated excellent resolution of pazopanib from its degradation products under forced degradation conditions, including acid/base hydrolysis, oxidation, photolysis, thermal, and humidity stress, confirming its stability-indicating capability. Validation in accordance with ICH Q2(R1) guidelines showed linearity over 25.71, 51.41, 82.26, 102.82, 123.38, and 154.23 µg/mL (r² > 0.999), precision with %RSD < 2.0, accuracy with recovery > 99%, and robustness under deliberate variations in chromatographic parameters. The limit of detection (LOD) and limit of quantification (LOQ) confirmed high sensitivity, and solution stability studies established analyte stability over 24 hours at 25 °C. With a runtime of 20 minutes and cost-efficient operation, this RP-HPLC method provides accurate, precise, and selective quantification of pazopanib in pharmaceutical dosage forms.
KEYWORDS- Pazopanib hydrochloride
- stability indicating RP-HPLC method
- forced degradation study
- assay
- analytical method validation