JOURNAL 3748
Journal of Chemical Metrology
Year: 2025 Issue: 2 July-December
p.199 - 208
Viewed 182 times.
GRAPHICAL ABSTRACT
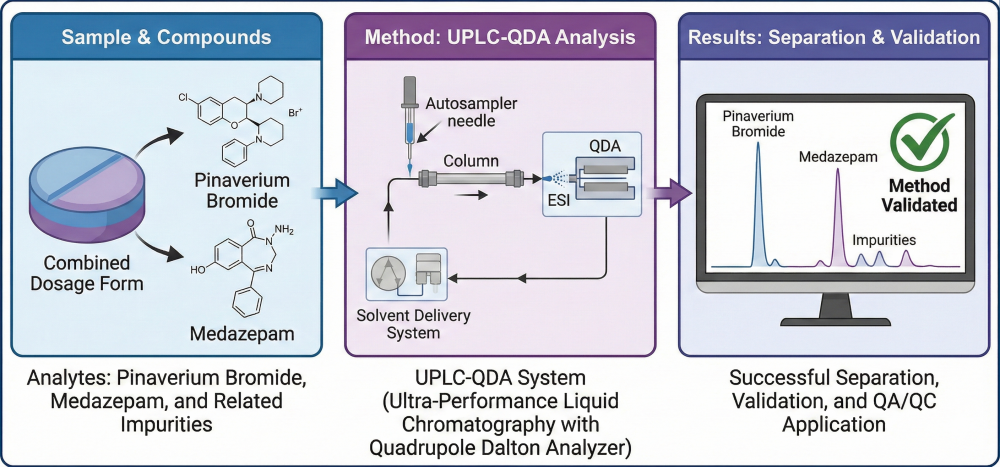
ABSTRACT
A rapid ultra-performance liquid chromatographic method coupled with a quadrupole detector (UPLC–QDA) was developed and validated for the simultaneous determination of pinaverium bromide, medazepam, and their related substances in a newly developed fixed-dose pharmaceutical formulation. Chromatographic separation was achieved on an Acquity BEH C18 column (100 × 2.1 mm, 1.7 µm) using a gradient elution of 25 mM ammonium formate buffer (pH 3.5) and acetonitrile at a flow rate of 0.6 mL/min and a column temperature of 45 °C. The total run time was 11 min, providing complete resolution of all analytes without interference. Linearity was demonstrated (R² ≥ 0.999) within the ranges of 2–400 ng/mL for medazepam, 10–2000 ng/mL for pinaverium bromide, 5–1000 ng/mL for RS1 (medazepam impurity), and 10–2000 ng/mL for RS3–RS6 (pinaverium bromide impurities). The limits of detection (LOD) and quantitation (LOQ) were 0.17 and 0.50 ng/mL for medazepam and 0.43 and 1.30 ng/mL for pinaverium bromide, respectively, while LOD and LOQ values for related substances ranged between 0.22–0.91 ng/mL and 0.66–2.70 ng/mL. The method was validated in accordance with the ICH Q2(R2) guideline. The validated method was successfully applied for the simultaneous quantification of pinaverium bromide, medazepam, and their respective impurities (RS1–RS6) in commercial film-coated tablets (50 mg/10 mg), demonstrating its applicability for routine quality control and stability testing of fixed-dose combination formulations.
KEYWORDS- Pinaverium bromide
- medazepam
- related impuriti
- UPLC–QDA
- method validation
SUPPORTING INFORMATION
Download File A8-128-JCM-2512-3748-SI.pdf (576.37 KB)