JOURNAL 1800
Organic Communications
Year: 2021 Issue: 1 January-March
p.48 - 57
Viewed 3462 times.
GRAPHICAL ABSTRACT
ABSTRACT
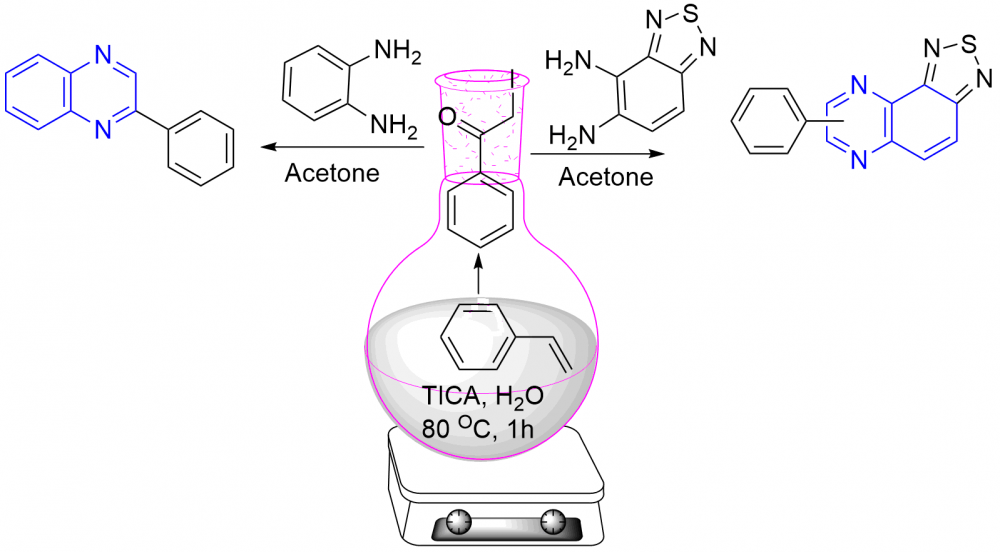
Triiodoisocyanuric acid (TICA) controlled one-pot and easy-operational protocol has been developed for the synthesis of substituted phenylquinoxalines (3a-3i) and phenyl-[1,2,5]thiadiazolo[3,4-f]quinoxaline (5a-5f) from styrenes with o-phenylenediamine and benzo[c][1,2,5]thiadiazole-4,5-diamine respectively. The reaction involves co-bromination and oxidation for the formation of an a-bromo ketone as an intermediate in the presence of triiodoisocyanuric acid, followed by condensation with the o-phenylenediamine and benzo[c][1,2,5]thiadiazole-4,5-diamine for the formation of phenylquinoxalines (3a-3i) and phenyl-[1,2,5]thiadiazolo[3,4-f]quinoxaline (5a-5f) in 55-79% yield. This protocol environmentally benign and economically viable. Substituted quinoxalines were obtained in good to excellent yields with wide substrate scope and functional-group tolerance.
KEYWORDS
- Quinoxalines
- one-pot protocol
- triiodoisocyanuric acid
- styrenes