JOURNAL 1069
Records of Natural Products
Year: 2020 Issue: 1 January-February
p.31 - 41
Viewed 4047 times.
GRAPHICAL ABSTRACT
ABSTRACT
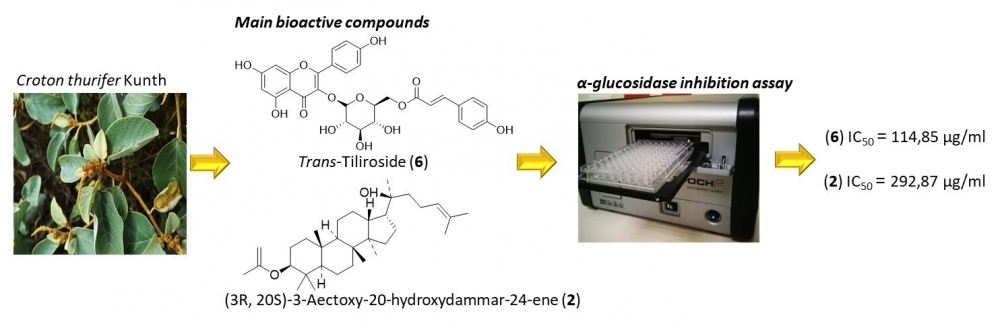
Phytochemical investigation of Croton thurifer, collected in Loja-Ecuador, led to the isolation of seven known compounds identified as: (3R, 20S) -3-palmitate-20-hydroxydammar-24-ene (1); (3R, 20S)-3-acetoxy-20-hydroxydammar-24-ene (2); trans-phytol (3); vomifoliol (4); β-sitosterol (5); trans-tiliroside (6) and sparsifol (7). The structures of the isolated compounds were determined by NMR, MS, as well as by comparison with literature data. The hypoglycemic activity of the crude extracts and isolated compounds was assessed by their ability to inhibit α-glucosidase activity. The hexane and methanol extracts did not show any inhibitory activity on α-glucosidase, while the ethyl acetate extract (EtOAc) showed very low inhibitory activity with IC50= 1.77 mg/mL. Two of the seven isolated compounds exhibited good inhibitory activity with IC50 values much higher than acarbose (377 μg/mL). trans-tiliroside was the compound with the highest inhibitory activity (IC50 = 114.85 μg / mL), while (3R, 20S)-3-Acetoxy-20-hydroxydammar-24-ene had moderate inhibitory activity (IC50=292.87). Trans-tiliroside exherted a non-competitive inhibition with Km values between 368 and 358 μM and Vmax value from 695 and 325 nM/min for unhibited and inhibited reaction, respectively and a Ki of 163,6 μM.
KEYWORDS- Croton thurifer
- α-glucosidase
- trans-tiliroside
- (3R, 20S)-3-Acetoxy-20-hydroxydammar-24-ene