Organic Communications
Year: 2020 Volume: 13 Issue:2 April-June
1) Evaluation of new 2-hydroxy-N-(4-oxo-2-substituted phenyl-1,3-thiazolidin-3-yl)-2-phenylacetamide derivatives as potential antimycobacterial agents
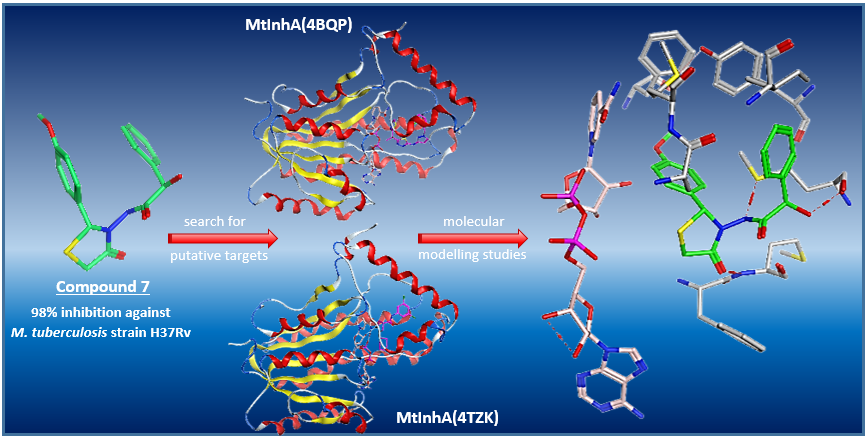
A small collection of 2-hydroxy-N-(5-methyl/nonsubstituted 4-oxo-2-substituted phenyl-1,3-thiazolidin-3-yl)-2-phenylacetamides (3-16) was synthesized from the cyclocondensation of 2-hydroxy-2-phenyl-N'-[(substitutedphenyl)methylene]acetohydrazides (2) and mercaptoethanoic acid or 2-mercaptopropanoic acid, characterized with spectral and elemental analysis. In order to explore their antimycobacterial potential, newly synthesized fourteen compounds were screened for their inhibitory activity against Mycobacterium tuberculosis strain H37Rv at 6.25 μg/mL with in-vitro primary tests. Compound 7 was found to provide the highest inhibition (98%) M. tuberculosis strain H37Rv, while most of the new derivatives showed different inhibition ratios. For the search of the putative targets which are considered as related to the antimycobacterial activity of these molecules, docking studies were performed. With molecular dynamic simulations, further possible interactions between ligands and the active site of the selected enzymes were investigated. Eventually, molecular modelling studies indicated that at least part of the mechanism of action of these compounds may be mediated by inhibition of MtInhA.
DOI http://doi.org/10.25135/acg.oc.78.20.05.1655 Keywords Mycobacterium tuberculosis 2-hydroxy-2-phenylacetohydrazide mercaptoethanoic acid 2-mercaptopropanoic acid 4-oxo-1,3-thiazolidines molecular modelling DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.2) N-Substituted aziridine 2-phosphonic acids and their antibacterial activities
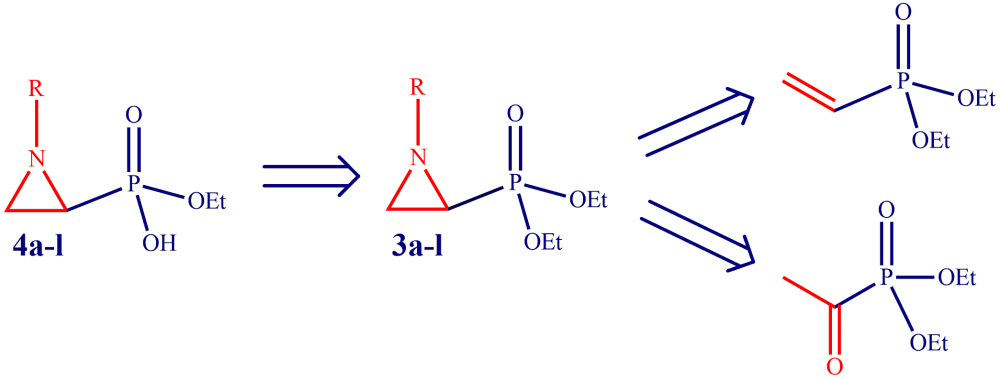
N-substituted aziridine diethyl phosphonates were synthesized easily in two steps starting from vinyl phosphonate or acetyl phosphonate. The controlled mono hydrolysis without opening the aziridine ring under mild reaction conditions was achieved by alkaline solution of LiOH. The corresponding phosphonic acid lithium salt was desalted by the use of Amberlite IRC-50H+ acting as an efficient and recyclable weakly acidic cation exchange promoter. Antimicrobial activity of the synthesized compounds was tested against E. coli ATCC 25922, Staphylococcus aureus (MRSA), Klebsiella pneumoniae NRLL B-4420, Acetobacter baumanii (wild type), Pseudomonas aeroginosa (ATCC 27853) and Enterobacter aerogenes NRLL B-3567. In general, all the compounds showed moderate antibacterial activity.
DOI http://doi.org/10.25135/acg.oc.77.20.03.1594 Keywords Aziridine-2-phosphonic acids atibacterial activity hydrolytic cleavage ion-exchange DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.3) Efficient synthesis and characterization of novel isoxazole derivatives including dihdyropyrazole and methanoisoindole moieties
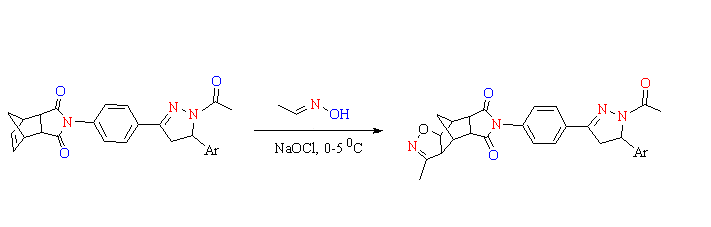
Isoxazole and pyrazole derivatives have a wide range of biological activity and they are of great interest to medicinal chemist. In this study, the synthesis and characterization of a series of novel hybrid molecules containing pyrazole and isoxazole rings ((3aS,4S,4aR,7aS,8S,8aS)-6-(4-(1-acetyl-5-(4-(aryl/heteroaryl))-4,5-dihydro-1H-pyrazol-3-yl)phenyl)-3-methyl-4,4a,8,8a-tetrahydro-3aH-4,8-methanoisoxazo-lo [4,5-f] isoindole-5,7(6H,7aH)-dione derivatives) were reported.
DOI http://doi.org/10.25135/acg.oc.79.20.06.1668 Keywords Isoxazole synthesis characterization NMR DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.4) Synthesis of chalcone-containing zinc and cobalt metallophthalocyanines; investigation of their photochemical, DPPH radical scavenging and metal chelating characters
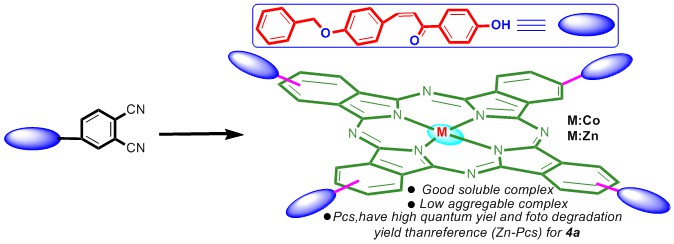
In this study, two new phthalocyanines (M = Zn and Co) were synthesized using the (E)-4-(4-(3-(4-(benzyloxy)phenyl)acryloyl)phenoxy)phthalonitrile (3) as ligand prepared from the chemical reaction of 4-nitrophthalonitrile with (E)-3-(4-(benzyloxy)phenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one (2). All compounds were characterized using by 1H-NMR, 13C-NMR, UV–Vis, FT-IR, and MALDI-TOF mass spectra. Singlet oxygen quantum yields of the synthesized compounds, aggregates in different solutions, metal chelating and 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging properties were reported.
DOI http://doi.org/10.25135/acg.oc.80.20.05.1639 Keywords Phthalocyanines photochemical studies singlet oxygen quantum yields metal chelating DPPH radical scavenging DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.