Records of Natural Products
Year: 2021 Volume: 15 Issue:5 September-October
1) Evidence-Based Medicinal Potential and Possible Role of Selaginella in the Prevention of Modern Chronic Diseases: Ethnopharmacological and Ethnobotanical Perspective

Different species of the genus Selaginella are exploited for various ethnomedicinal purposes around the globe; mainly to cure fever, jaundice, hepatic disorders, cardiac diseases, cirrhosis, diarrhea, cholecystitis, sore throat, cough of lungs, promotes blood circulation, removes blood stasis and stops external bleeding after trauma and separation of the umbilical cord. Though, high content of various phytochemicals has been isolated from Selaginella species, flavonoids have been recognized as the most active component in the genus. Crude extract and different bioactive compounds of this plant have revealed various in vitro bioactivities such as, antimicrobial, antiviral, anti-diabetic, anti-mutagenic, anti-inflammatory, anti-nociceptive, anti-spasmodic, anticancer and anti-Alzheimer. However, more studies into the pharmacological activities are needed, since none of the professed bioactivity of this plant have ever been fully evaluated. Therefore, this review aims to discuss the evidence-based ethnomedicinal and ethnopharmacological uses, phytochemicals and bioactive potential of Selaginella species. It will provide an updated knowledge for ethnobotanists, ethnopharmacologists and other scientific communities to rethink over the possible usage of Selaginella in medicine. Moreover, further explorations are needed to formulate a novel medicinal product from Selaginella extracts for the improvement of human health, together with toxicity evaluations, necessary to ensure about the safety of these medicinal lycophytes.
DOI http://doi.org/10.25135/rnp.222.20.11.1890 Keywords Selaginella chronic diseases anti-Alzheimer anti-diabetic ethnobotany phytochemistry DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.2) Coumarins from Angelica dahurica and Their Antitumor Activities in Human MG-63 Osteosarcoma Cells
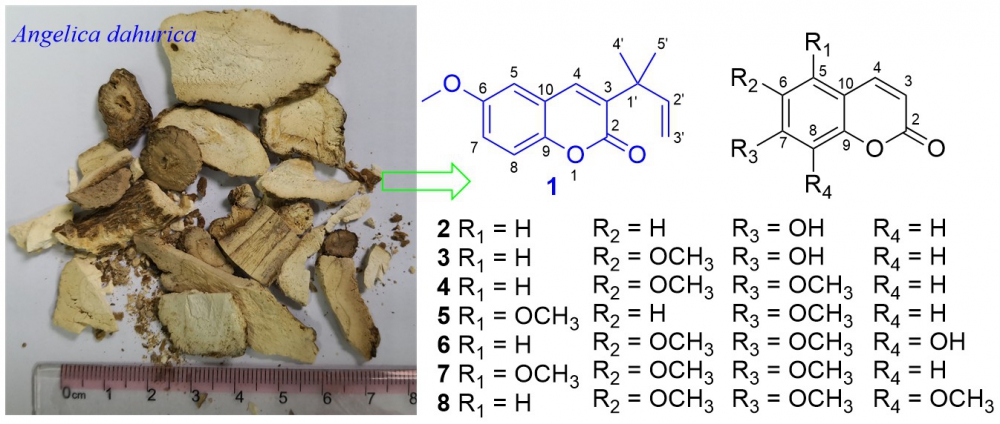
A new coumarin, angedahurin A (1), and seven known analogues (2−8), were isolated from the roots of Angelica dahurica. Their structures were identified by extensive NMR, IR, and HR-ESIMS spectroscopic analyses. The cytotoxicities of coumarins 1−8 against MG-63 human osteosarcoma cell lines were screened. Compound 1 showed significant cytotoxic effects against MG-63 with an IC50 value of 7.2 uM,for comparison, the positive control, 5-FU, had an IC50 value of 32.4 uM. Morphological features of apoptosis activities were evaluated in 1-induced MG-63 cells and the results confirmed MG-63 cell apoptosis in a dose-dependent manner.
DOI http://doi.org/10.25135/rnp.225.21.01.1935 Keywords Angelica dahurica coumarin apoptosis cytotoxicity DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.3) A New Cyclic Tetrapeptide from Endophytic Fungus Aspergillus versicolor E-2
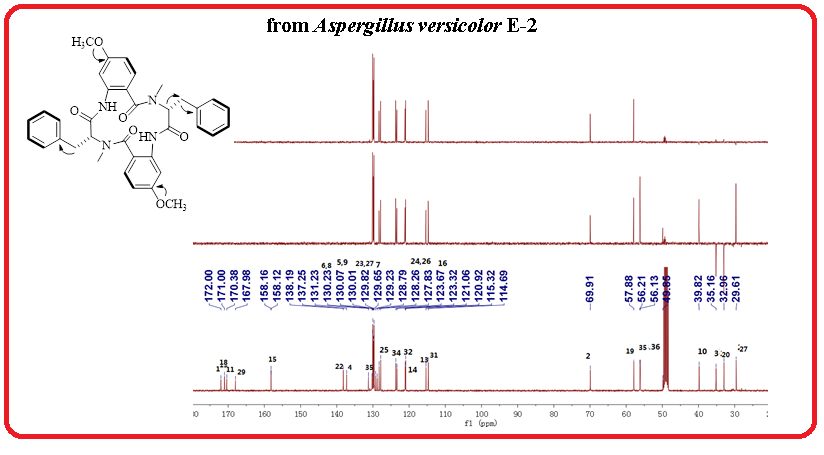
A new cyclic tetrapeptide (1) named aspergilpeptide A, together with a known cyclic tetrapeptide penicopeptide A (2) and chaetominine (3) were obtained from the endophytic fungus Aspergillus versicolor E-2 isolated from the medicinal plant Euphorbia royleana. The structures of compounds (1-3) were elucidated using NMR and MS methods.
DOI http://doi.org/10.25135/rnp.225.21.01.1931 Keywords Cyclic tetrapeptide endophytic fungus Aspergillus versicolor DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.4) New Selective Human MAO-B Inhibitors from the Stems of Erythrina corallodendron L.
One new, 10, 11-dioxo-6,7α-erythraline epoxide (A1) and four known erythrinan alkaloids 10, 11-dioxo-erythraline (A2), erysodine (A3), 8-oxo-erythraline (A4) and erythraline (A5) were isolated from the 70% methanolic extract of stems of E. Corallodendron (Fabaceae). The isolated compounds were elucidated by exploiting 1D/2D NMR, and HR-ESI-MS analysis. The absolute configuration of A1 was determined by electronic circular dichroism (ECD). Mono Amine Oxidase inhibitory activity of the isolated alkaloids was investigated in vitro using kynuramine deamination assay on recombinant human MAO-A and B enzymes. The binding modes were predicted by molecular docking and the structure activity relationships of erythrinans were then evaluated. All isolated alkaloids demonstrated preferential activity against MAO-B. A1 displayed the highest potency and selectivity against MAO-B with IC50 of 25.18 μM, (SI >3.97). The selective inhibition exhibited by erythrinan alkaloids against MAO-B is in line with the expected biological impact of Erythrina in the treatment of neurodegenerative diseases and presents this chemical class as promising leads for managing AD and PD diseases.
DOI http://doi.org/10.25135/rnp.229.21.01.1940 Keywords Erythrina corallodendron alkaloids Alzheimer’s disease Parkinson’s disease selective MAO-B inhibitors docking DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.5) Isolation and Characterization of Glycosidic Tyrosinase Inhibitors from Typhonium giganteum Rhizomes
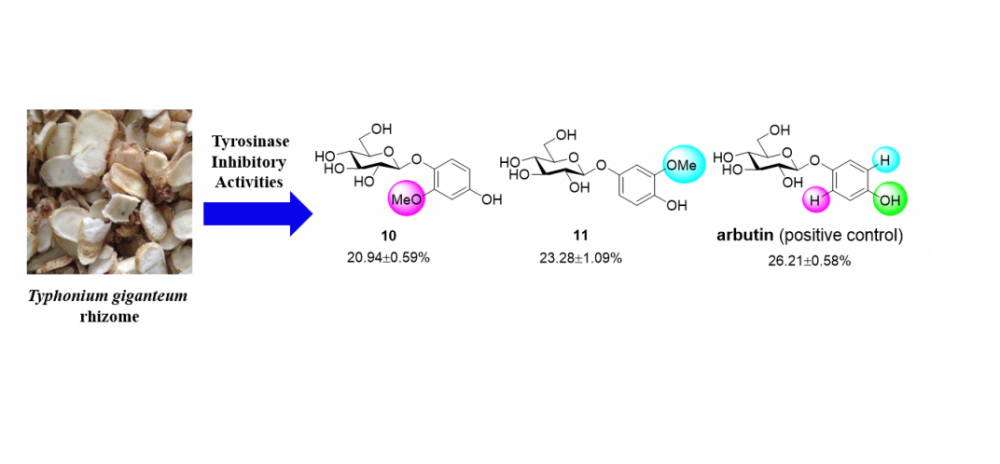
A new hydrocinnamoyl glucoside, 1-O-(4-hydroxyhydrocinnamoyl)-β-D-glucopyranose (1), together with fifteen known glycosides, including two phenylethanoid glycosides (2–3), two cinnamoyl glycosides (4–5), six phenolic glycosides (6–11), one lignan glycoside (12) and four megastigmane glycosides (13–16) were isolated from a 95% EtOH extract of the Typhonium giganteum rhizomes. The sixteen glycosides were structurally characterized by NMR, HRESIMS, enzymatic hydrolysis and comparison with literature. Upon evaluating inhibitory activities of compounds 1–16 against mushroom tyrosinase at 25 μM, compounds 10 and 11 exhibited obvious inhibitory activities, with %inhibition values of 20.94±0.59%, 23.28±1.09%, respectively, with arbutin used as the positive control (26.21±0.58%).
DOI http://doi.org/10.25135/rnp.230.21.02.1965 Keywords Typhonium giganteum tyrosinase inhibitor glycoside arbutin DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.6) Characterization of Nepeta viscida, N. nuda subsp. nuda and the Putative Hybrid N. × tmolea Essential Oils
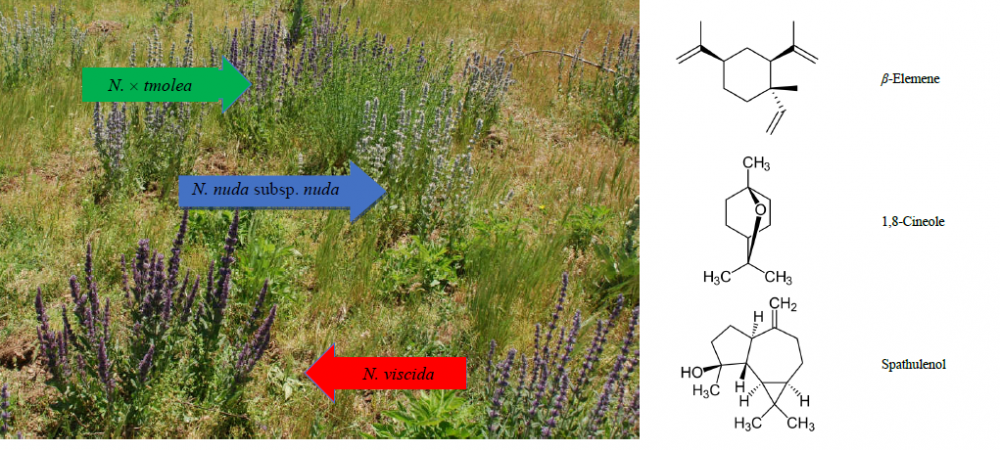
Nepeta viscida, N. nuda subsp. nuda, and their putative hybrid N. × tmolea were evaluated for their essential oils with chemotaxonomical aspect. Hybrid of N. x tmolea individuals were observed in the regions where the distribution of N. viscida and N. nuda subsp. nuda taxa were present, namely Dursunbey (Balıkesir) and Ödemiş (İzmir) natural habitats, respectively. The aerial parts of the taxa were hydrodistilled for 4 h using a Clevenger-type apparatus. The essential oils were analyzed both by gas chromatography-mass spectrometry (GC/MS) and gas chromatography flame ionization detector (GC-FID). The main components of the oils for the species collected were spathulenol, β-elemene, and 1,8-cineole, supporting the hybridization proposition.
DOI http://doi.org/10.25135/rnp.250.21.04.2033 Keywords Essential oil Nepeta viscida Nepeta nuda subsp. nuda Nepeta × tmolea chemotaxonomy DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.7) Dicentrine and Dicentrinone Isolated from Stephania tetrandrae Radix Suppress HepG2 Proliferation through Inhibiting PDI Activity
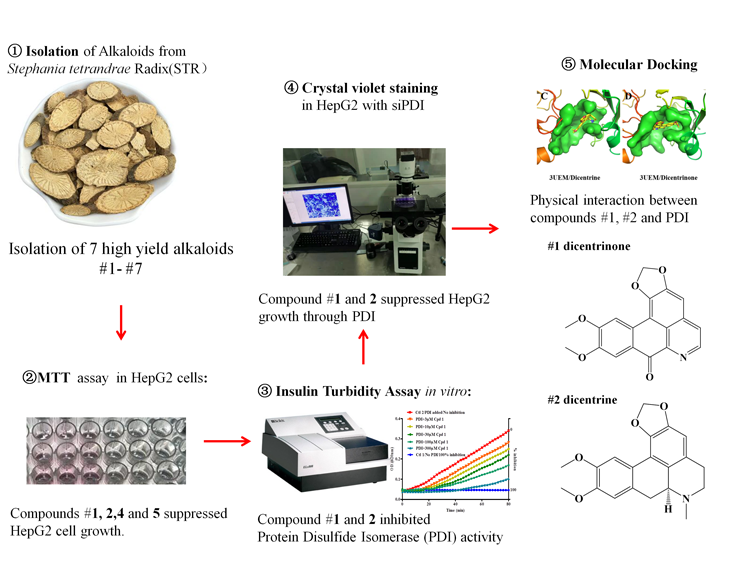
Inhibition of protein disulfide isomerase (PDI) has been attempted as a promising anti-cancer strategy. However, there is still no currently available PDI inhibitors approved for clinical use. Here, we isolated seven high yield alkaloids from Stephaniae tetrandrae Radix (STR), a medical herb frequently prescribed in anti-tumor condition, and identified two potent natural PDI inhibitors, dicentrine and dicentrinone. Among the seven alkaloids isolated, dicentrinone (1), dicentrine (2), tetrandrine (4), and fangchinoline (5) could significantly reduce cell viability in a dosage dependent manner detected by MTT assay in human hepatoma cells. To examine whether the candidate compounds are potent PDI inhibitors, we performed insulin turbidity assay and found dicentrine and dicentrinone, but not tetrandrine and fangchinoline, could effectively inhibit PDI activity, with IC50 of 56.70 μM and 43.95 μM respectively. Meanwhile, dicentrine and dicentrinone failed to further reduce the cell number index when co-treated with siRNA of PDI, suggesting the compounds behave as PDI inhibitors. Furthermore, dicentrinone and dicentrine have been successfully docked to the active pocket of PDI (PDB #3UEM) by molecular docking, suggesting the existence of physical interaction between compounds and PDI. Our results suggested that dicentrine and dicentrinone may be developed into safe PDI inhibitors.
DOI http://doi.org/10.25135/rnp.231.21.01.1929 Keywords Stephania tetrandrae Radix Dicentrine Dicentrinone HepG2 PDI DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.8) Jatroidaine A: a New Tetranortirucallane Type Triterpene from Jatropha multifida
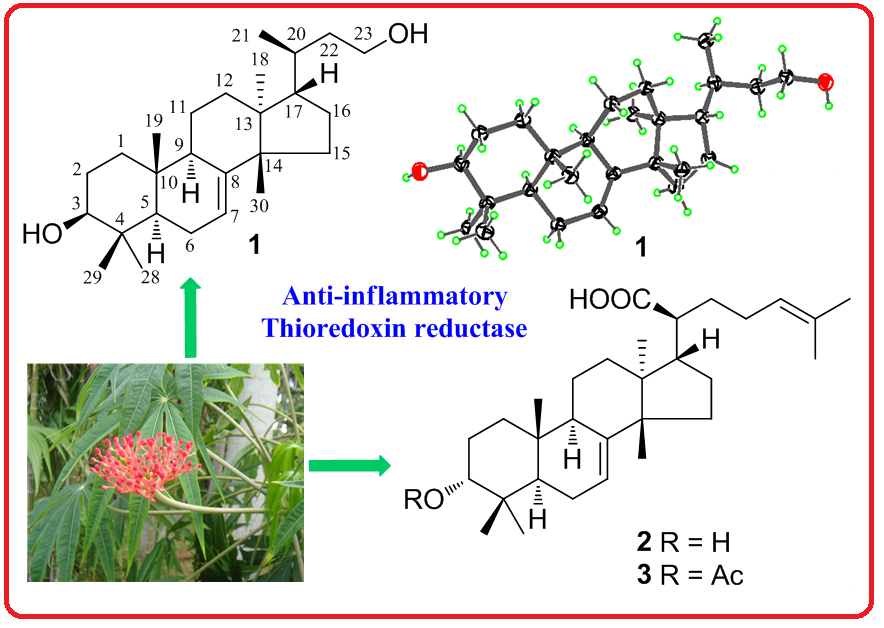
Jatroidaine A (1), a new tetranortirucallane-type triterpene, and two known analogues (2−3) were isolated from the leaves and branches of Jatropha multifida. Their structures were fully elucidated by extensive spectroscopic methods and comparison to known compounds. The absolute configuration of 1 was assigned by single-crystal X-ray diffraction analysis. All compounds were evaluated for their anti-inflammatory and thioredoxin reductase (TrxR) inhibitory activities. Unfortunately, no significant activity was observed.
DOI http://doi.org/10.25135/rnp.233.21.02.1968 Keywords Jatropha multifida tirucallane triterpene anti-inflammatory activity thioredoxin reductase DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.9) Cyclic Polyketides with α-Glucosidase Inhibitory Activity from Endiandra kingiana Gamble and Molecular Docking Study
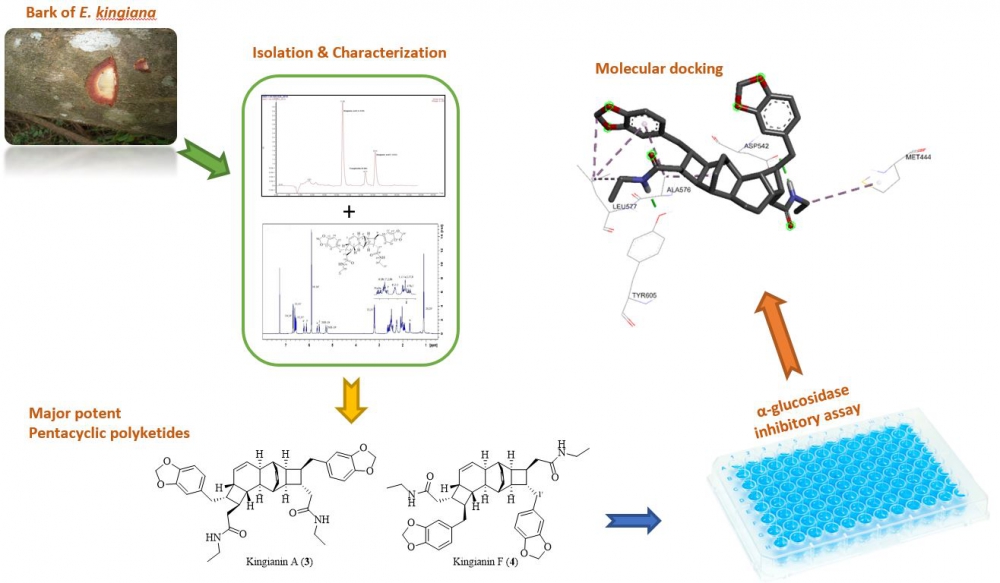
A phytochemical investigation of the methanolic extract of Endiandra kingiana (bark) led to the isolation of four major compounds which are kingianic acid A (1), tsangibeilin B (2), kingianin A (3) and kingianin F (4). The structures were determined by 1D- and 2D-NMR analysis in combination with HRMS experiments. The compounds were screened for their in vitro α-glucosidase inhibition activity. Among them, compounds 3-4 showed potent α-glucosidase inhibition activity with IC50 value at 11.9 ± 2.0 µM and 19.7 ± 1.5 µM, respectively. The molecular docking study found that both compounds were bound into the active site of the N-terminal of MGAM, and thus agreed with the in vitro α-glucosidase enzyme inhibition activity results.
DOI http://doi.org/10.25135/rnp.227.20.11.1889 Keywords Endiandra kingiana cyclic polyketides Endiandric acids kingianins α-glucosidase inhibition molecular docking DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.