JOURNAL 3745
Bioorganic and Medicinal Chemistry Reports
Year: 2025 Issue: 2 July-December
p.28 - 53
Viewed 9 times.
GRAPHICAL ABSTRACT
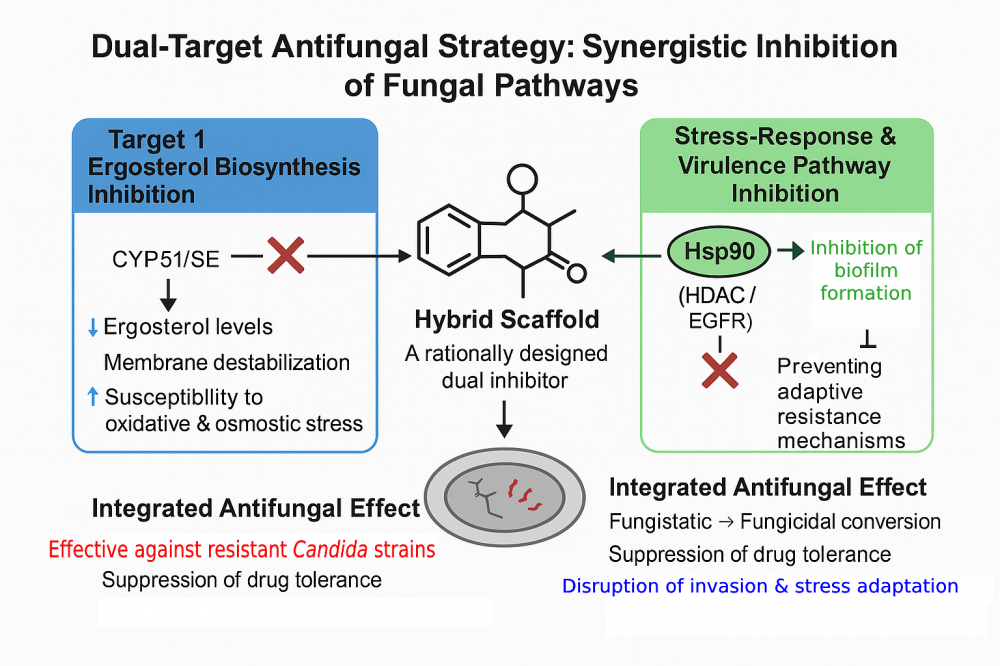
ABSTRACT
Global climate change, the increasing prevalence of immunosuppressed patient populations, and mounting environmental selection pressures have collectively enhanced the adaptive capacity of pathogenic fungi (most notably Candida albicans) transforming invasive fungal infections into a major global clinical threat. The limited chemical diversity and narrow target spectrum of current antifungal agents, particularly those acting through a unidirectional mechanism centered on CYP51 inhibition, facilitate the rapid emergence of drug resistance. C. albicans simultaneously activates a network of interconnected resistance mechanisms to survive antifungal exposure. CYP51 point mutations and gene amplification, overexpression of efflux pumps, HSP90–calcineurin–dependent stress adaptation, and epigenetic reprogramming mediated by HDAC/HAT imbalance constitute the core components of this defensive architecture. Moreover, the central regulatory role of the EGFR–MAPK axis in epithelial invasion underscores that C. albicans pathobiology extends far beyond metabolic pathways, relying instead on the coordination of a sophisticated signaling network. This multilayered defense system inherently restricts the efficacy of single-target antifungal agents. Consequently, combination therapies that simultaneously engage distinct biological pathways hold the potential to enhance antifungal efficacy through pharmacodynamic synergy. However, substantial variability in clinical responses, drug–drug interactions and limited translational evidence have hindered the routine implementation of combination regimens in clinical practice. These constraints have positioned dual-target antifungal design as a more rational and feasible alternative. Notably, clinical evidence supports multi-axis antifungal engagement, with late-stage pipelines advancing toward novel resistance-relevant targets such as the Phase 3 Gwt1 inhibitor fosmanogepix. Recent studies on dual active architecture, including CYP51 and HDAC, HSP90 and HDAC, CYP51 and SE, as well as CYP51 and HSP90, identify four core axes governing C. albicans fitness: ergosterol biosynthesis, epigenetic regulation, proteostatic stress response, and epithelial invasion. Coordinated modulation of these axes results in synergistic suppression of antifungal resistance and virulence. In this review, we provide an integrated evaluation of the molecular foundations of antifungal resistance in C. albicans, the pharmacodynamic advantages of combination therapies, and the therapeutic promise of dual-target design strategies. Collectively, the evidence supports multi-target antifungal strategies as a transformative paradigm capable of achieving durable, resistance-agnostic efficacy.
KEYWORDS- Invasive fungal infection
- histone deacetylase
- ergosterol
- lanosterol 14α-demethylase
- dual inhibitors