JOURNAL 2717
Organic Communications
VOLUME & ISSUE
Year: 2023 Issue: 1 January-March
Year: 2023 Issue: 1 January-March
PAGES
p.35 - 45
p.35 - 45
STATISTICS
Viewed 2257 times.
Viewed 2257 times.
GRAPHICAL ABSTRACT
ABSTRACT
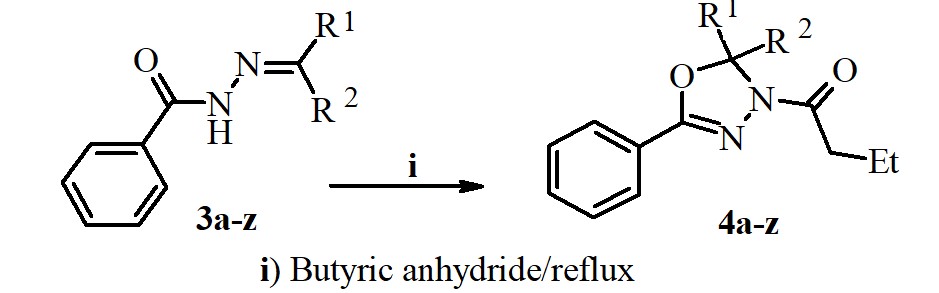
A new series of N-butyryl-1,3,4-oxadiazolines were synthesized via oxidative cyclization reaction of different benzoyl hydrazones with butyric anhydride. The structures of obtained compounds were confirmed by IR, MS, 1H NMR, 13C NMR and Elemental analysis methods and are in full agreement with their molecular structure. The synthesized 1,3,4-oxadiazolines were screened for in vitro for their biological activity against a variety of bacterial strains (Euterococci, Escherichia coli, Staphylococcus aureus, Klebsiella spp, Proteus spp, and fungi (Aspergillus niger, Candida albicans), employing the nutrient agar disc diffusion method. The obtained results showed that these compounds have good inhibition against the tested pathogens.
KEYWORDS- 1,3,4-Oxadiazolines
- N-acylhydrazones
- spiro-oxadiazole
- oxidative cyclization