JOURNAL 2127
Records of Natural Products
VOLUME & ISSUE
Year: 2022 Issue: 4 July-August
Year: 2022 Issue: 4 July-August
PAGES
p.324 - 334
p.324 - 334
STATISTICS
Viewed 1448 times.
Viewed 1448 times.
GRAPHICAL ABSTRACT
ABSTRACT
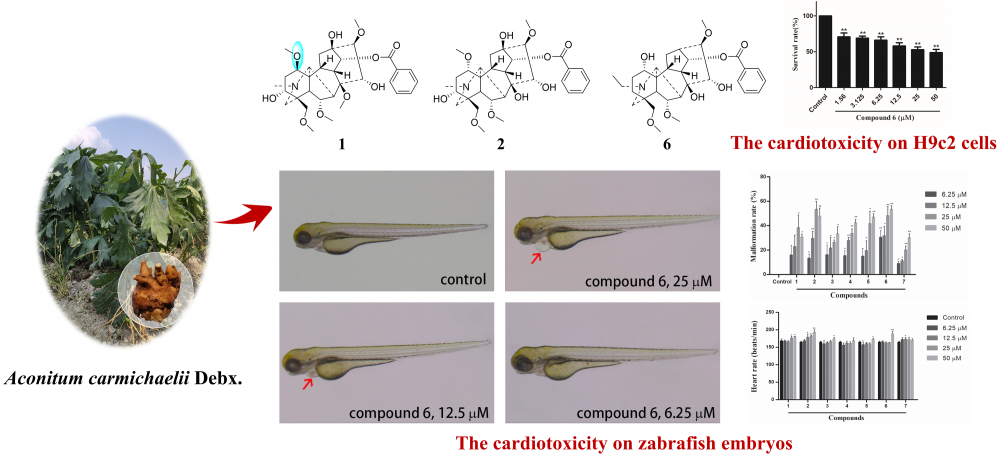
A new (1) and six known (2-7) monoester-type C19-diterpenoid alkaloids were isolated from the lateral roots of Aconitum carmichaelii Debx. Their structures were determined by spectroscopic techniques and calculations of NMR chemical shifts. Compound 1 (1-epi-hokbusine A) was an aconitine-type diterpenoid alkaloid possessing an unusual 1β-methoxy group. According to the main toxicity of the lateral roots of A. carmichaelii, the cardiotoxic effects of the isolates were evaluated using H9c2 rat myocardial cells and zebrafish embryos. The results showed that all the monoester-type C19-diterpenoid alkaloids exhibited cardiotoxicity, and compound 6 was found to be the most toxic compound.
KEYWORDS- Aconitum carmichaelii
- monoester-type C19-diterpenoid alkaloids
- H9c2 cells
- zebrafish embryos
- cardiotoxicity