JOURNAL 3145
Records of Natural Products
Available Online: April 08,2024
p.1 - 8
http://doi.org/10.25135/rnp.452.2402.3145 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 201 times.
-
Ngoc-Hong Nguyen

-
Boi-Phong Vuong

-
Chuong Hoang Nguyen

-
A Dong Huynh

-
Dinh-Manh Nguyen

-
Thuc-Huy Duong

-
Hoang-Thanh Kpa

-
Thi-Kim-Dung Le

-
Thi-Ngoc-Duyen Nguyen

-
Huy Truong Nguyen

-
Jirapast Sichaem

GRAPHICAL ABSTRACT
ABSTRACT
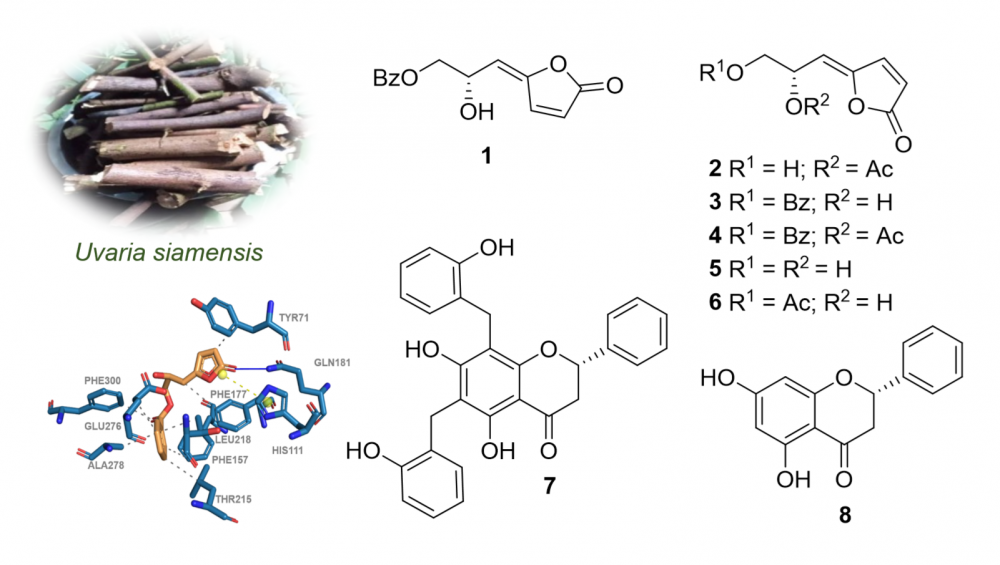
A new γ-butenoid, (Z)-6-acetylsphaerodol (2), along with seven known compounds, (4E)-7-benzoyloxy-6-hydroxy-2,4-heptadien-4-olide (1), (6S)-melodorinol (3), (6S)-acetylmelodorinol (4), (Z)-sphaerodiol (5), (Z)-7-acetylsphaerodol (6), dichamanetin (7), and pinocembrin (8), were isolated and structurally elucidated from Uvaria siamensis growing in Thailand. Their chemical structures were assigned by extensive spectroscopy (NMR and HRESIMS), as well as comparisons with the previous literature. Compounds 1-8 were evaluated for the antimicrobial activity against two multidrug-resistant Staphylococcus aureus strains, 23Sa1 and SA-ATCC. Compounds 4 and 6 displayed moderate activity with inhibitory zones of 13 and 12 mm against 23Sa1 and SA-ATCC strains, respectively. Compounds 3 and 7 exhibited significant efficacy, displaying IC50 values of 157.7 and 94.2 µM, respectively, against α-glucosidase. According to the docking data, compounds 3 and 7 have a greater influence on binding contacts with the α-glucosidase active pocket, thereby affecting the inhibitory activity of the enzyme.
KEYWORDS- Annonaceae
- Uvaria siamensis
- (Z)-6-acetylsphaerodol
- γ-butenoid
- antibacterial activity
- α-glucosidase inhibition