JOURNAL 2693
Journal of Chemical Metrology
Year: 2023 Issue: 1 January-June
p.15 - 24
Viewed 2640 times.
GRAPHICAL ABSTRACT
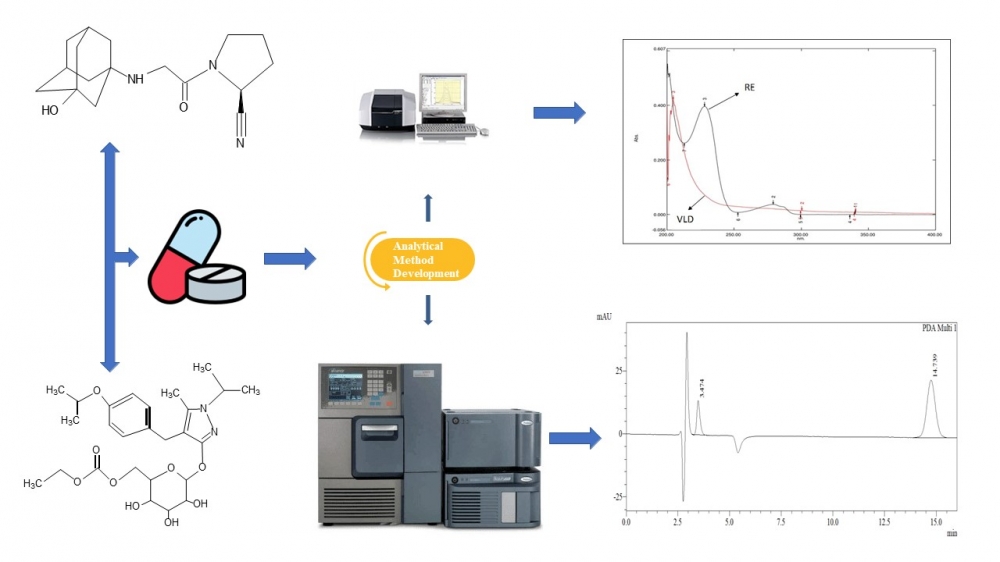
ABSTRACT
The ultraviolet spectrophotometric and reversed-phase high-performance liquid chromatography methods were developed and validated to quantify remogliflozin etabonate and vildagliptin in a fixed-dose tablet formulation. The materials and methods used in this study involved using methanol as the solvent in developing three ultraviolet spectrophotometric techniques, namely simultaneous equation, absorption ratio, and derivative spectroscopy. The wavelength maxima for vildagliptin and remogliflozin etabonate were determined to be 232 nm and 217 nm, respectively. The chromatographic method involved using a Phenomenex Luna C18 column (250 × 4.6 mm, 5 µm) and a mobile phase consisting of a mixture of methanol and acetate buffer (pH 5.6) in a 70:30 ratio. The flow rate was set at 1 mL/min, and the detecting wavelength was 210 nm. The results obtained from the optimized approaches met the performance test criteria set by the International Conference on Harmonization guideline Q2 (R1). The methods were then applied to assay marketed tablet formulations, and the results obtained were within acceptable limits. In conclusion, the developed techniques can be used to analyze the fixed-dose tablet formulation regularly
KEYWORDS- FDC
- Remogliflozin Etabonate
- HPLC-PDA
- T2DM
- vildagliptin