Records of Natural Products
Year: 2021 Volume: 15 Issue:6 November-December (Special Issue Dedicated to the Memory of Professor Ayhan Ulubelen)
1) Special Issue Dedicated to the Memory of Professor Ayhan Ulubelen (1931-2020)

Prof. Dr. Ayhan Ulubelen who is one of the pioneers and a world-wide recognized authority in Natural Products Chemistry, has passed away on 29 November 2020 in Istanbul. This issue is dedicated to the memory of Professor Ayhan Ulubelen (1931-2020)
DOI http://doi.org/10.25135/rnp.255.21.05.2021 Keywords Ayhan Ulubelen Obituary DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.2) Biosynthesis of the Ommochromes and Papiliochromes
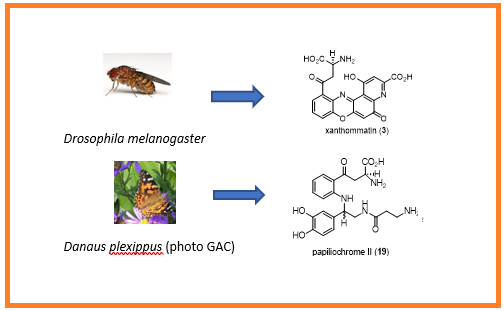
A brief overview of the biosynthesis of the ommochromes and the papiliochromes is presented.
DOI http://doi.org/10.25135/rnp.238.21.02.1988 Keywords Ommochromes Papiliochromes Alkaloids L-Tryptophan Dopamine Biosynthesis DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.3) Protective Effects of Natural Products and Their Derivatives on Genetic Material: A Critical Review
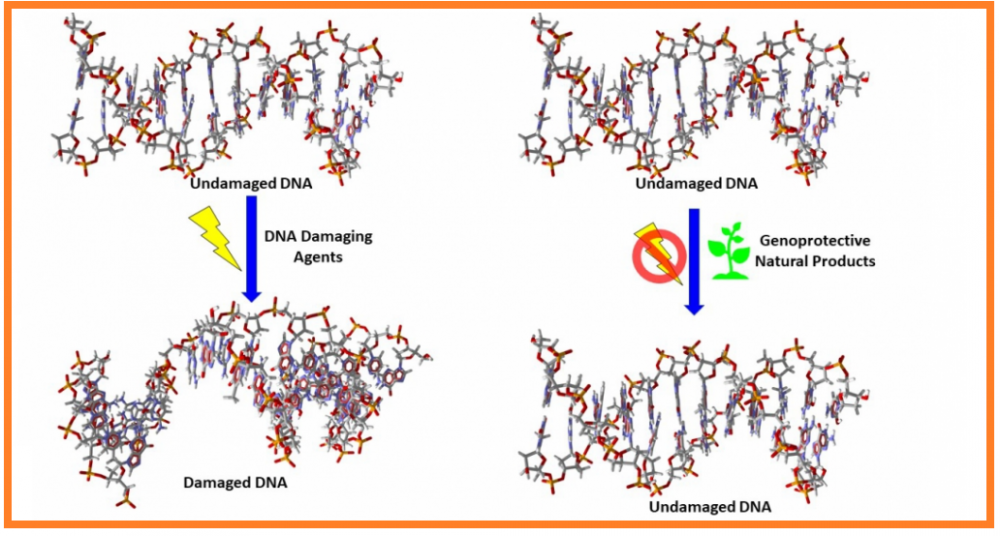
Although treatment with natural products and the substances derived from them has gained much attention, it is important to know the genomic safety of these substances prior to their use in humans. The present review aims to present the current knowledge on the genoprotective effects and possible mechanism of actions of natural compounds. Therefore, an up-to-date search was conducted using known databases such as PubMed, ScienceDirect, and Clinicaltrials.gov. For the investigation of genotoxic/genoprotective activity of these substances, comet or micronucleus assay were frequently used models applied through eukaryotic test systems, bacterial strains, cultured animal cells or tissues (e.g., mice, rats) but also human by using oxidizing or carcinogenic agent-induced DNA damage capacity. Findings suggest that several extracts, including those from medicinal plants, marine algae or their preparations, antioxidants such as quercetin, retinoids, resveratrol, hyaluronic acid, carnosol, rosmarinic acid, and naringin have shown genoprotective effects in various test systems. Antioxidant, anti-inflammatory, mitogenic, reduction of DNA strand breaks and DNA lesions, formation of micronucleus, and chromosomal aberrations were the observed mechanisms of action of genoprotective substances. In conclusion, this review highlights the importance of natural products, especially dietary antioxidants, which can be safely used for the treatment of various diseases.
DOI http://doi.org/10.25135/rnp.228.20.11.1871 Keywords Antioxidants extracts genoprotective effects Comet assay DNA damage DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.4) Di- and Triterpenoids Isolation and LC-MS Analysis of Salvia marashica Extracts with Bioactivity Studies
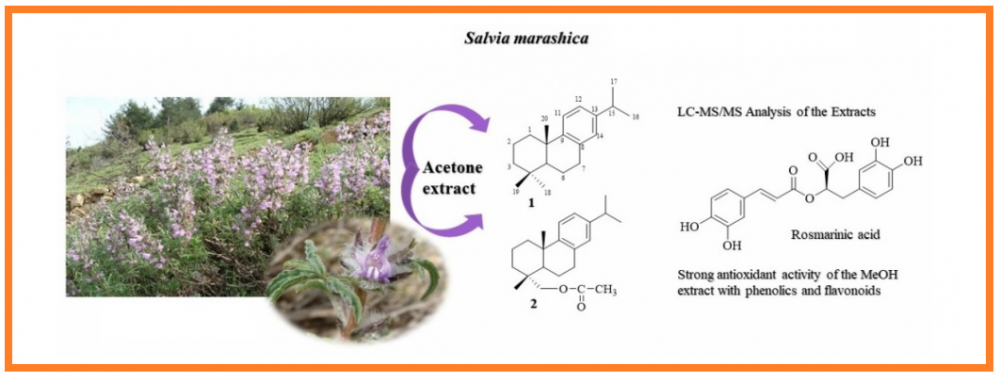
In this study, dichloromethane, acetone, and methanol extracts of the aerial parts of the Salvia marashica plant which is an endemic species to Anatolia, were investigated. The total phenolic amounts of these extracts were determined as pyrocatechol equivalent and total flavonoids as quercetin equivalent. Antioxidant activity was determined by four complementary methods including inhibition of lipid peroxidation (by β-carotene color expression), DPPH free radical scavenging activity, ABTS cation radical scavenging activity and CUPRAC methods. Anticholinesterase activity of the extracts was investigated by the Ellman method against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes. Viability and cytotoxic activity tests were carried out on the fibroblast L929 cells and cytotoxic A549 lung cancer cells, respectively. The triterpenoids and diterpenoids constitute the major secondary metabolites of the S. marashica acetone and methanol extracts isolated by chromatographic methods. Their structures were determined based on spectroscopic methods, namely NMR and mass analyses. Ten terpenoids were obtained from either acetone or methanol extracts of the S. marashica. Seven of them were triterpenoids, elucidated as lupeol, lupeol-3-acetate, lup-12, 20(29)-diene, lup-20(29)-ene, α-amyrin-tetracosanoate, oleanolic acid and ursolic acid besides a steroid β-sitosterol. Two abietane diterpenes, abieta-8,11,13-triene (1) and 18-acetoxymethylene-abieta-8,11,13-triene (2), were obtained from the acetone extract which were isolated from a Salvia species for the first time in the present study. The methanol extract was found to be very rich in rosmarinic acid determined by LC-MS/MS analysis.
DOI http://doi.org/10.25135/rnp.251.21.03.2003 Keywords Salvia marashica terpenoids flavonoids NMR LC-MS/MS bioactivity DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.5) Study of Anacyclus pyrethrum Lag. Root Extract against Aedes aegypti Linn. Larvae: Potential Vector Control for Dengue Viral Fever
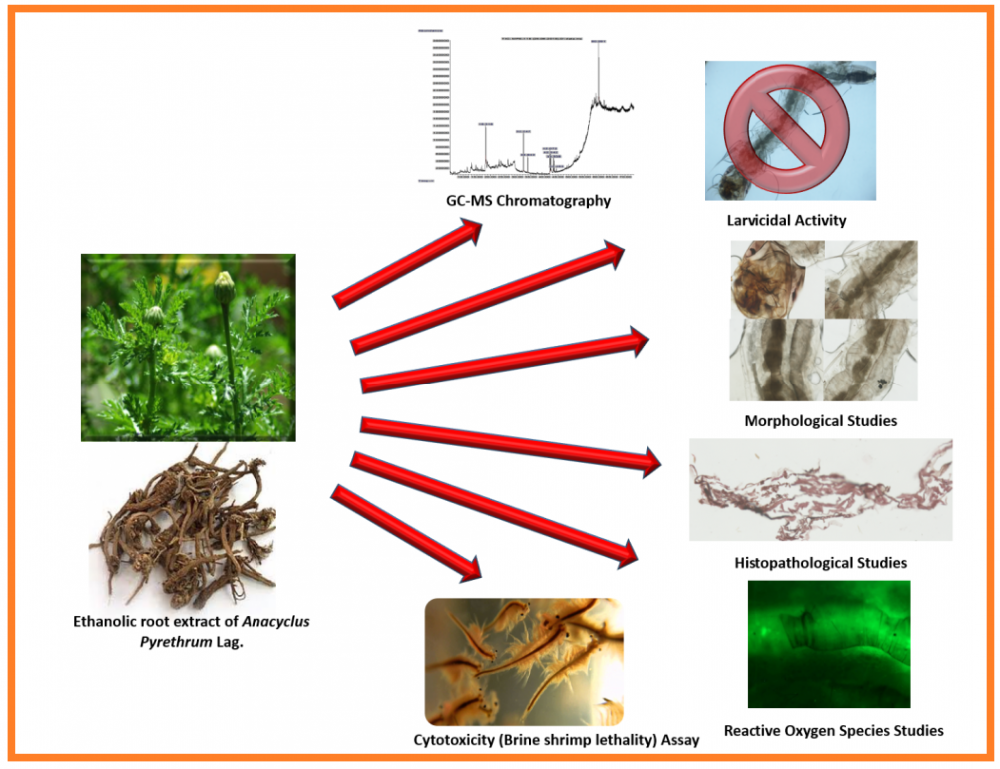
Anacyclus pyrethrum Lag. is a medicinal plant. In this study we evaluated the susceptibility of larvae of Aedes aegypti Linn. against potential larvicidal effects of root extract of A. pyrethrum. Bioassays were performed using World Health Organization methodologies. GC-MS analysis was employed to determine the contents of the extract. The underlying mechanisms of larvicidal activity were analyzed by using morphological, histological, and Reactive Oxygen species (ROS) studies. The GC-MS of A. pyrethrum root extract showed nine compounds. During initial screening, the root extract killed 90% of 3rd instar larvae of A. aegypti at 0.2 mg/mL concentration as compared to standard permethrin. Different larval stages exhibited 100% larvicidal activity, especially more naive 1st and 2nd instar larvae. The dose dependent larvicidal activity against 3rd instar larvae, showed reduced effect with decreased concentration. Disruption in gut line of 3rd instar larvae is proposed to be mediated from the ROS production, evident in fluorescent oxidant sensitive assay. The extract exhibited non-cytotoxic profile in brine shrimp lethality assay with IC50 of 126.93 ± 0.3 µg/mL as compared to standard etoposide i.e., IC50 = 7.46 ± 0.05 μg/mL. The study inferred that the root extract of A. pyrethrum can serve as promising biopesticide for the control of A. aegypti, which is a vector of many viral diseases.
DOI http://doi.org/10.25135/rnp.243.21.02.1979 Keywords Anacyclus pyrethrum Lag. Aedes aegypti Linn. larvicidal Activity ovicidal activity dengue hemorrhagic fever biopesticides DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.6) Secondary Metabolites from Teucrium creticum L.
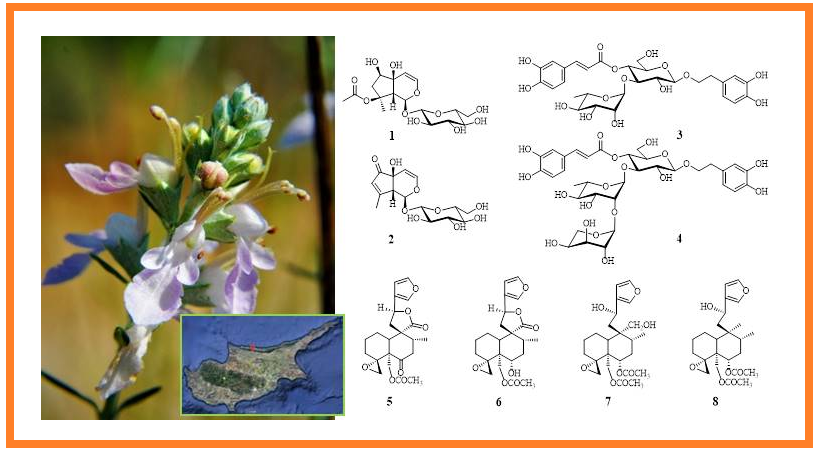
From the aerial parts of Teucrium creticum L. (Lamiaceae) eight compounds, 1 – 8 were isolated using chromatographical methods. Based on the results of spectroscopical analysis such as UV, 1D-NMR (1H-, 13C-NMR, DEPT-135), 2D-NMR (COSY, HSQC, HMBC, NOESY) and HRMS, the structure of the compounds were determined as two iridoids, 8-O-acetylharpagide (1) and teuhircoside (2), two phenylethanoid glycosides, verbascoside (= acteoside) (3) and lavandulifolioside (4), and four neoclerodane-type diterpenoids, teucrin H3 (= 19-acetylgnaphalin) (5), teucjaponin B (6), teucretol (7) and diacetylteumassilin (8).
DOI http://doi.org/10.25135/rnp.232.20.12.1922 Keywords Lamiaceae Teucrium creticum iridoids phenylethanoid glycosides neo-clerodane-type diterpenoids. DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.7) Chemical Composition and Cytotoxic Effect of Prangos turcica A. Duran, M. Sagiroglu & H. Duman
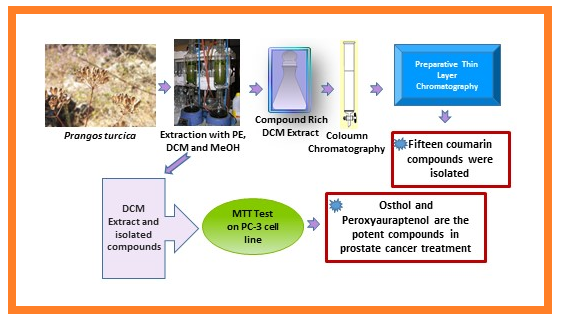
In addition to the antiflatulent, emollient, antifungal, antihemorrhoidal, antioxidant, anthelmintic effects, Prangos species have been used to stop bleeding and for the treatment of wounds and scars in central Asia and Turkey. In the present study, the compounds were isolated using chromatographic methods, and their structures were identified by 1H NMR and direct comparison with the reference compounds where available. Fifteen known coumarins were isolated from the dichloromethane extract as osthol, murraol, auraptenol, peroxyauraptenol, 4'-senecioiloxyosthol, meranzin hydrate, scopoletin, umbelliferone, isoimperatorin, oxypeucedanin, oxypeucedanin hydrate, oxypeucedanin methanolate, gosferol, psoralen, and marmesin. The cytotoxic activities of all isolated compounds from dichloromethane extract of P. turcica roots were evaluated using MTT assay on human adenocarcinoma (prostate PC-3) cells. 4'-senecioiloxyosthol, oxypeucedanin methanolate, gosferol, psoralen, peroxyauraptenol and marmesin were tested for the first time on the PC-3 cell line. Osthol and peroxyauraptenol showed the highest cytotoxic activity with IC50 values of 65 and 72 µg/mL, respectively. Additionally, auraptenol, scopoletin, gosferol, psoralen, 4'-senecioiloxyosthol and dichloromethane extract of root part (Pt/R/DCM) demonstrated moderate to low cytotoxic activity. Consequently, the most potent compounds, osthol and peroxyauraptenol, may be used as a lead compound to develop effective drug substances to treat prostate cancer.
DOI http://doi.org/10.25135/rnp.235.21.02.1991 Keywords Coumarin derivatives Prangos turcica cytotoxic activity DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.8) Anticholinergic, Antidiabetic and Antioxidant Activities of Ferula orientalis L. Determination of Its Polyphenol Contents by LC-HRMS
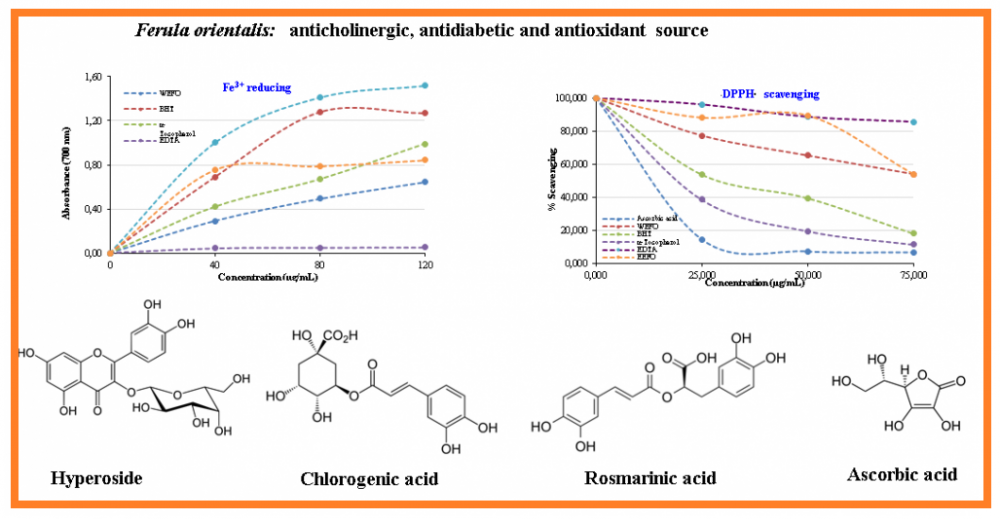
To evaluate the antioxidant activity of evaporated ethanolic extract of Ferula orientalis L. (EEFO) and lyophilized water extract of Ferula orientalis L. (WEFO) several in vitro antioxidant methods such as ABTS•+ scavenging activity, DPPH· scavenging activity, Fe3+reduction method, cupric ions (Cu2+) reduction capacity, and metal ion (Fe2+)-binding activities using ferrozine reagent were separately performed. Also, BHT, α-tocopherol and ascorbic acid were used as the standard antioxidant molecules. Moreover, some phenolic compounds that are responsible for antioxidant abilities of EEFO and WEFO were determined by LC-HRMS. EEFO and WEFO demonstrated effective antioxidant abilities when compared with the standards. EEFO demonstrated IC50 values of 1.946 µg/mL against acetylcholinesterase (AChE), 0.815 µg/mL against α-glycosidase, and 0.675 µg/mL against α-amylase.
DOI http://doi.org/10.25135/rnp.236.21.02.1983 Keywords Ferula orientalis α-glycosidase antioxidant activity phenolic compound acetylcholinesterase α-amylase DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.9) Cytotoxic Sesquiterpene Coumarins from the Roots of Heptaptera cilicica
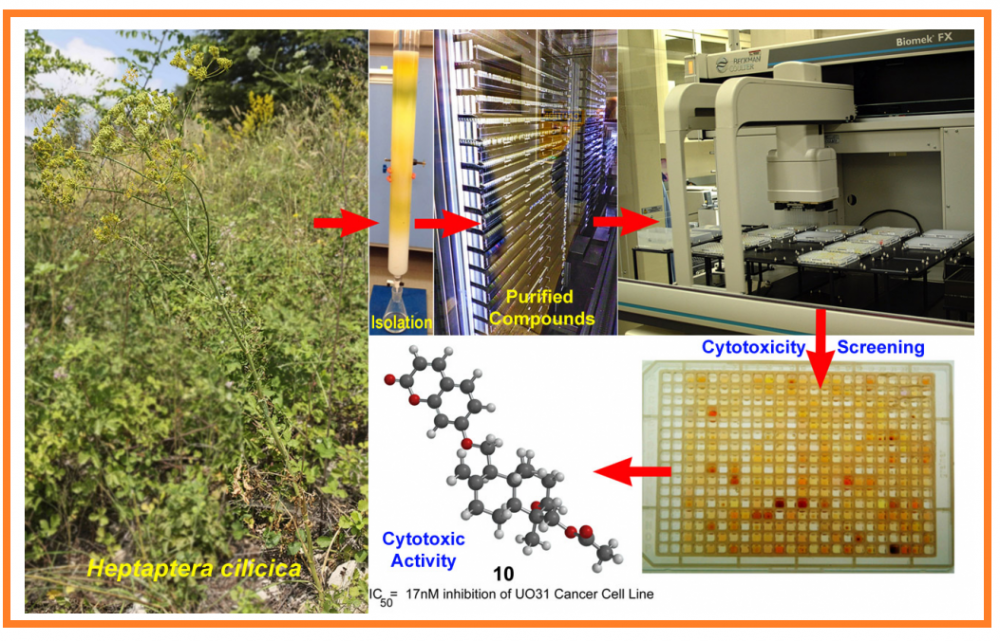
Preliminary 2-day cancer cell screening assays indicated that the dichloromethane extract of the roots of Heptaptera cilicica showed relatively strong cytotoxic activity. Eleven sesquiterpene coumarin ethers with potent and selective cytotoxic activities were isolated from the dichloromethane extract of the roots of H. cilicica. Furthermore, revised spectroscopic data for 14′-acetoxybadrakemone (4) and complete NMR spectroscopic assignments for colladonin (6) and colladin (7) are reported.
DOI http://doi.org/10.25135/rnp.242.21.02.1990 Keywords Heptaptera cilicica Apiaceae sesquiterpene coumarins cytotoxic activity DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.10) Saponins from Twenty-Two Cephalaria Species
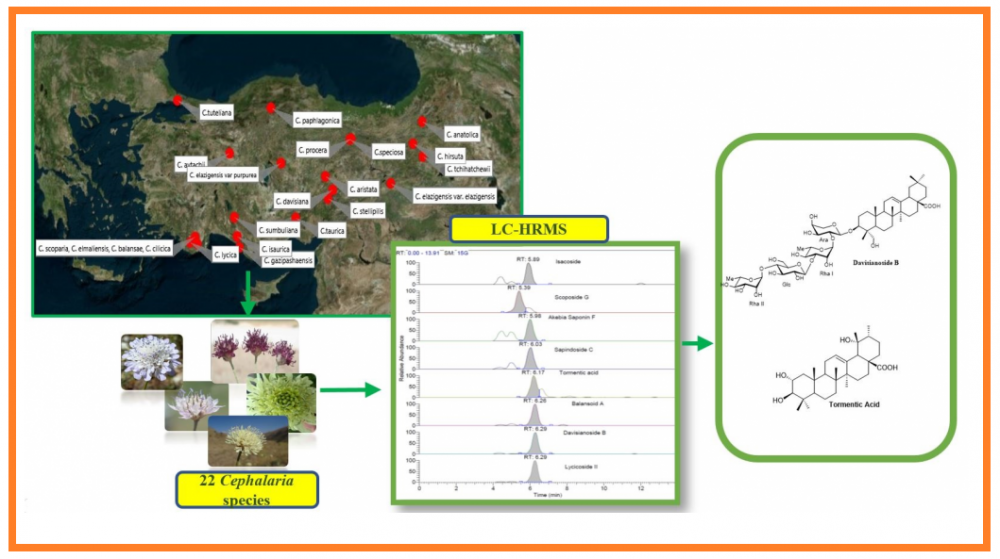
Saponins in the Cephalaria species have a broad spectrum of biological properties and application potentials. This research aimed to develop a novel method to tentatively identify and quantify triterpenes and saponins in 22 Cephalaria species by LC-HR/MS method. In this study, we provided a broad screening of important biologically active triterpenes and saponins with high separation ability, high sensitivity and high selectivity. This is the first report to prove that Cephalaria species can be a good source of tormentic acid which has many biological activities in literature. The main triterpene compound, tormentic acid was presented at the level between 74.02 mg/kg and 75702.0 mg/kg in n-butanol extract of Cephalaria species. The source of some biologically active saponins such as davisianoside B, aristatoside C, elmalienoside A, balansoide B and scoposide B were also determined with this study, for the first time. Therefore, due to the different activities and usages of the identified compounds, these findings may find application in a number of industries such as nutraceutical, pharmaceutical, medical and cosmetic industries.
DOI http://doi.org/10.25135/rnp.241.21.02.1985 Keywords Cephalaria Caprifoliaceae triterpene saponin validation LC-HR/MS DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.11) Antimicrobial, Cytotoxic, Antiviral Effects, and Spectroscopic Characterization of Metabolites Produced by Fusarium oxysporum YP9B
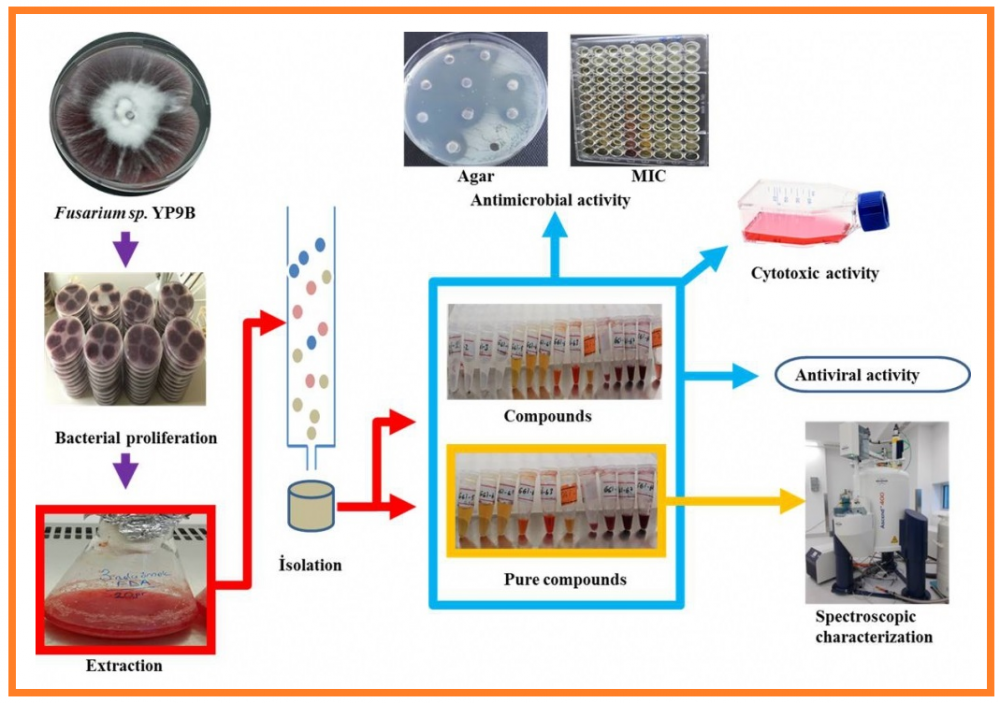
The goal of the work is to determine the bioactive pharmaceutical metabolites produced by the Fusarium oxysporum YP9B isolate. Ten new natural compounds were isolated from the ethyl acetate extract of the F. oxysporum YP9B strain. Their structures were elucidated by spectroscopic methods using 1D and 2D NMR, UV, FT-IR, and mass spectra (LC-QTOF MS and GC-FID/MS). Identified compounds were named as; (1-benzyl-2-methoxy-2-oxoethyl)-2-hydroxy-3-methylbutanoate (1), 2-oxo-8-azatricyclo[9.3.1.13,7]-hexadeca-1(15),3(16),4,6,11,13-hexaen-10-one (2), 2,3-dihydroxypropanoic, hexadecanoic anhydride (3a), 2,3-dihydroxypropanoic (9Z)-octadecenoic anhydride (3b), 2,3-dihydroxy-propanoic (9Z,12Z)-octadecadienoic anhydride (3c), 2,3-dihydroxypropanoic (11Z)-octadecenoic anhydride (4a), 2,3-dihydroxypropanoic, (9E,12E)-octadecadienoic anhydride (4b), 3-hydroxy-1,2,6,10-tetramethylundecyl hexzadecanoate (5a), 3-hydroxy-1,2,6,10-tetramethylundecyl (9E)-octadecaenoate (5b), and 3-hydroxy-1,2,6,10-tetramethylundecyl octadecanoate (5c). Antimicrobial activities of the isolates obtained from the YP9B strain were determined. Cytotoxic and antiviral activities were tested for the isolates against VERO, MCF-7, PC-3, and A549. Compounds 5a-c, 1, and 3a-c showed bacteriostatic activity at low concentrations, and 4a-b and 2 were found to be bactericides. MIC and MBC values against Mycobacterium smegmatis for the compounds 5a-c and 1 were determined to be <0.5 µg/mL and 0.46 µg/mL, respectively. The experimental result showed that compounds 2, 5a-c and 1 have strong cytotoxic (7.51±1.38 and 19.13± 0.68 (µM) IC50) activity. The antiviral activity against HSV type-1 was determined to be 1.25 µM for compounds 4a-c and 0.312 µM for compound 1.
DOI http://doi.org/10.25135/rnp.208.20.06.1674 Keywords Fusarium oxysporum YP9B seconder metabolite antimicrobial cytotoxic antiviral DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.12) Biological and Chemical Comparison of Natural and Cultivated Samples of Satureja macrantha C.A.Mey.
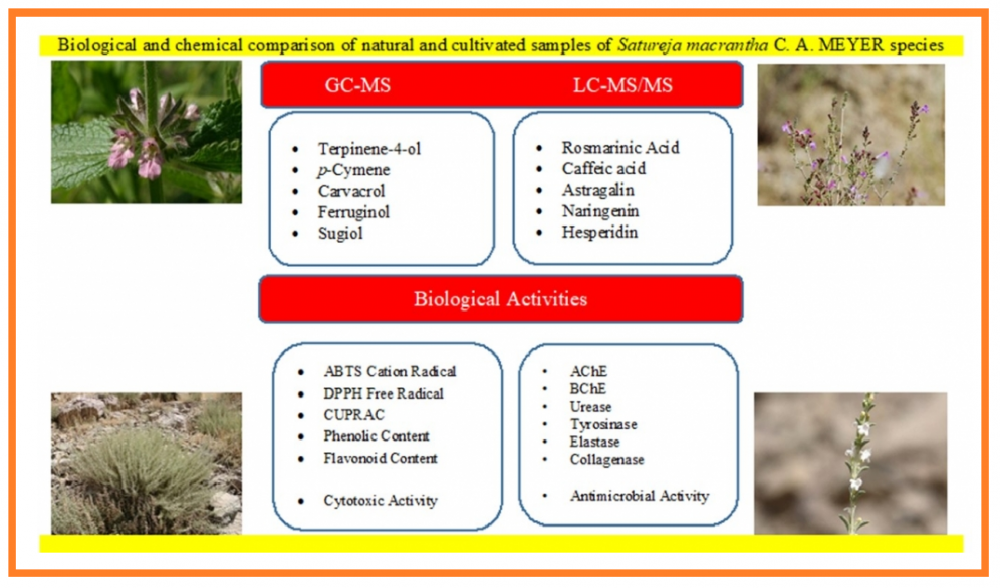
In this study, investigation on the essential oils and ethanol extracts of naturally grown and cultivated Satureja macrantha samples were reported. The essential oil, flavour and terpenoid-steroid-flavonoid contents of S. macrantha samples were determined by GC-MS and their phenolic contents by LC-MS/MS. Besides, the biological activities of the samples were investigated for their antioxidant, anti-Alzheimer, antimicrobial, cytotoxic, antityrosinase, antiurease, antielastase and anticollagenase properties. The phenolic content and antioxidant capacity of the cultivated sample were higher than those of the naturally grown sample. According to the GC-MS results, terpinene-4-ol (30.9%) and p-cymene (56.7%) were determined as the major components in the essential oils of the naturally grown and cultivated S. macrantha, respectively. The flavour analysis results showed that cis-sabinene hydrate (20.7%) and carvacrol (42.2%) were found to be the major components in the naturally grown and cultivated samples, respectively. While the naturally grown sample was rich in abietane diterpenoids (ferruginol (17.5 mg analyte/g extract) and sugiol (4.2 mg analyte/g extract)), these components were not detected in the cultivated sample. The rosmarinic acid content (0.20 and 24.87 mg analyte/g extract, respectively) of the cultivated sample was found to be significantly higher than that of the natural sample. The biological activities of the samples were determined to be changed in parallel with their chemical contents that are due to factors such as climatic conditions, and soil structure.
DOI http://doi.org/10.25135/rnp.237.21.02.1957 Keywords Satureja macrantha essential oil flavour terpenoid content biological activities spectroscopic analyses DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.13) Bioguided Isolation of Secondary Metabolites from Salvia cerino-pruinosa Rech. f. var. cerino-pruinosa
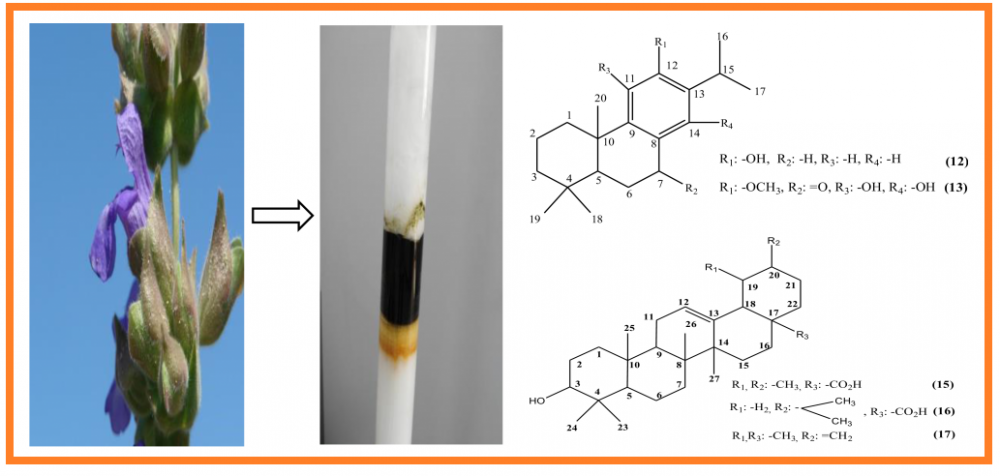
In the current study, the ethanol extracts prepared from the aerial parts and roots of an endemic species, Salvia cerino-pruinosa Rech. f. var. cerino-pruinosa were fractionated on silica gel columns and tested for determination of their antioxidant activity using DPPH free radical and ABTS cation radical scavenging, and cupric reducing antioxidant capacity (CUPRAC) test assays. Twenty known secondary metabolites were isolated from the active antioxidant fractions; rosmarinic acid (1), chlorogenic acid (2), caffeic acid (3), 4-hydroxybenzoic acid (4), benzoic acid (5), luteolin 7-O-glucoside (6), bis-(2-ethylhexyl)benzene-1,2-dicarboxylate (7), salvianolic acid A (8), salvianolic acid B (9), 7-acetylroyleanone (10), 6,7-dehydroroyleanone (11), ferruginol (12), inuroyleanol (13), 12-hydroxy-6,7-secoabieta-8,11,13-triene-6,7-dial (14), ursolic acid (15), oleanolic acid (16), taraxasterol (17), lupenone (18), β-sitosterol (19), and stigmasterol (20). Rosmarinic acid, which was obtained from the aerial parts, was found to be the best antioxidant compound among the isolated secondary metabolites in DPPH free radical and ABTS cation radical scavenging, and CUPRAC assays (IC50: 1.20±0.04 µg/mL, IC50: 1.74±0.06 µg/mL, A0.5: 1.22±0.02 µg/mL, respectively). Chlorogenic and caffeic acids, luteolin 7-O-glucoside, salvianolic acids A and B, and inuroyleanol exhibited also high antioxidant activity in the mentioned assays.
DOI http://doi.org/10.25135/rnp.248.21.01.1933 Keywords Salvia cerino-pruinosa isolation secondary metabolite antioxidant DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.14) Microbial Transformation of (–)-α-Bisabolol Towards Bioactive Metabolites
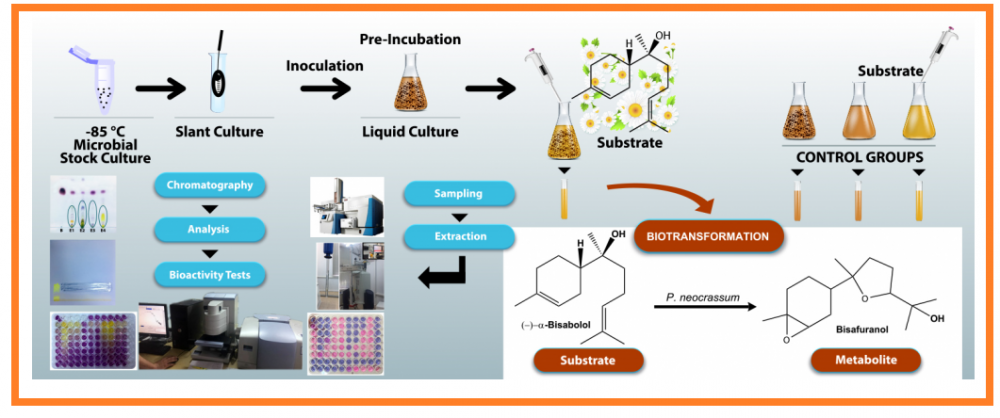
Bisabolol is one of the bioactive constituents of chamomile. It was aimed to biotransform (–)-α-bisabolol by different fungi for the production of new bioactive metabolites, which was converted to α-bisabolol oxide A and B by Thamnidium elegans. Additionally, the biotransformation by Penicillium neocrassum yielded a new metabolite, which was characterized as 2-(5-methyl-5-(6-methyl-7-oxabicyclo[4.1.0]heptan-3-yl)tetrahydrofuran-2-yl)propan-2-ol = bisafuranol. The substrate and its metabolite mixtures were tested using antioxidant DPPH• scavenging assay. Antimicrobial activity was evaluated by an in vitro microdilution assay against a panel of human pathogenic bacteria and yeasts resulting in the lowest MIC and MFC values of 150 μg/mL. (–)-α-Bisabolol was effective against Propionibacterium acnes and Staphylococcus epidermidis (75, 37.5 μg/mL, respectively). The antioxidant activity of the metabolites was found to be more effective in scavenging free radicals.
DOI http://doi.org/10.25135/rnp.252.2004.1615 Keywords microbial transformation Matricaria recutita L. bisabolol antimicrobial antioxidant Penicillium neocrassum DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.15) Benzodiazepine Derivatives from Marine-Derived Streptomyces cacaoi 14CM034
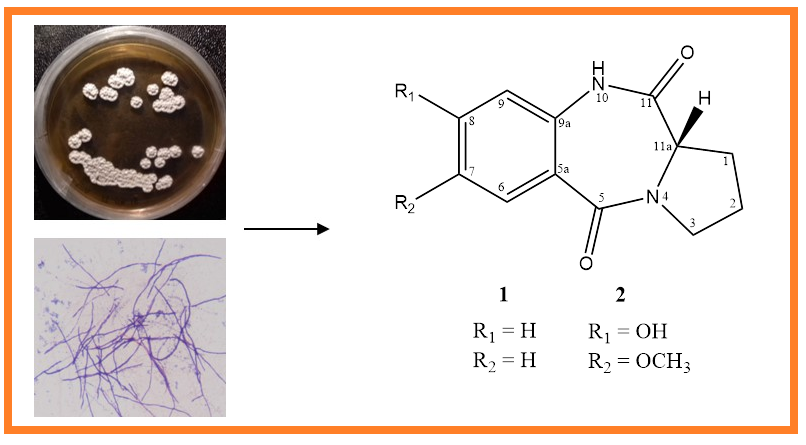
7-methoxy-8-hydroxy cycloanthranilylproline (2), a new natural product with pyrrolobenzodiazepine (PBD) framework, was isolated from marine-derived actinobacterium Streptomyces cacaoi 14CM034, together with cycloanthranilylproline (1). Structural elucidation of the compounds was based on FTIR, 1D- (1H and 13C NMR), 2D-NMR (COSY, HMBC and NOESY) and HR-MS analyses. Compounds 1 and 2 exhibited notable antimicrobial activity. The presence of PBD derivatives in S. cacaoi was first demonstrated with this study.
DOI http://doi.org/10.25135/rnp.203.20.08.1766 Keywords Marine actinobacterium Streptomyces cacaoi Benzodiazepine Antimicrobial activity Antibiotics DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.16) Bioactive Natural Products from Endophytic Fungus Aspergillus nidulans Associated with Nyctanthes arbor-tristis Linn.
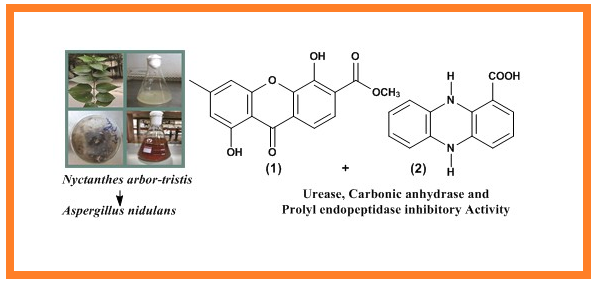
Endophytic fungi are a substantial source of bioactive secondary metabolites. Present studies on Aspergillus nidulans―an endophytic fungus from Nyctanthes arbor-tristis Linn. afforded one new 1, 5-dihydroxy-3-methylxanthone-6-carboxylic acid methyl ester (1) and one known 5, 10-dihydro-phenazine-1-carboxylic acid (2) compound. The structures were elucidated by spectral techniques (Mass, 1D and 2D NMR), and their urease, carbonic anhydrase and prolyl endopeptidase inhibitory activities were evaluated. Compound 1 showed notable prolyl endopeptidase inhibition (IC50 = 3.13 ± 0.09 µM) and compound 2 showed significant carbonic anhydrase (IC50 =14.3 ± 1.05 µM) and urease (IC50 = 24.3 ± 1.15 µM) inhibition.
DOI http://doi.org/10.25135/rnp.251.21.02.1986 Keywords Aspergillus nidulans; Nyctanthes arbor-tristis; xanthone DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.17) Eudesmane Sesquiterpenoids from Salvia plebeia
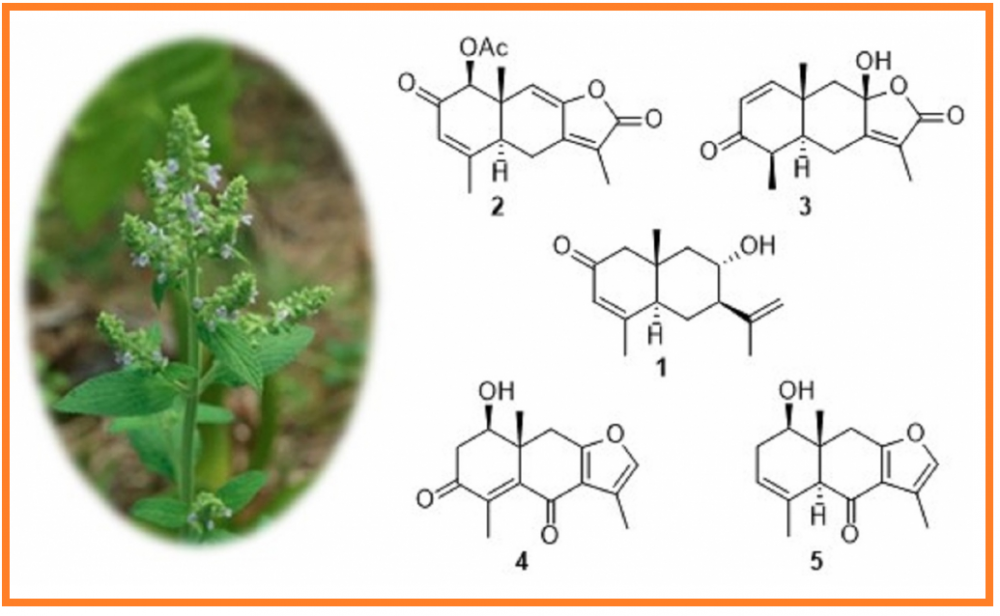
A new eudesmane sesquiterpenoid (1), named sapleudesone, together with four known analogs (2-5) were isolated from the aerial parts of Salvia plebeia. The structure of compound 1 was established by NMR and HRESIMS data, and the absolute configuration of 1 was determined by comparing the experimental ECD spectrum with the calculated ECD spectra. The known compounds were identified to be salplebeone A (2), linderolide I (3), chlorantene D (4), and chlomultin B (5), respectively, by comparing the NMR data and specific rotations with reported data. All five compounds were tested for the inhibitory effects against NO production in LPS-activated RAW 264.7 macrophages. As a result, compound 2 exhibited weak inhibitory effects with an IC50 value of 42.3 ± 1.4 mM.
DOI http://doi.org/10.25135/rnp.218.20.10.1856 Keywords eudesmane sesquiterpene Salvia plebeia; NO production inhibitory effects DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.