Records of Natural Products
A scientific open access journal in the field of natural products.LATEST ARTICLES
A New Sesquiterpene from the Fungus Penicillium sp. LPFH-hzw-zw1
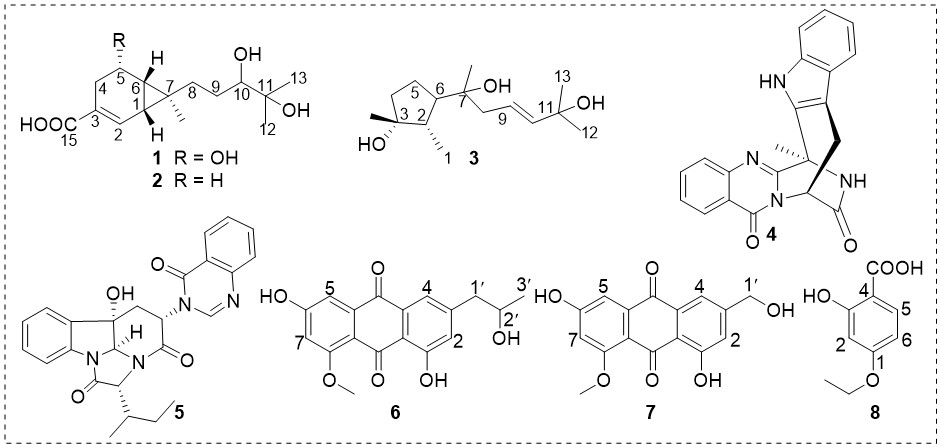 An investigation into the secondary metabolites of the sea sediment-derived Penicillium sp. LPFH-ZW-1 led to the isolation of a new compound, named penicipeneacid (1), along with seven known compounds. The structures of these compounds were identified through extensive analysis of the 1D and 2D NMR data, combined with HRESIMS data. The absolute configuration of 1 was determined by comparing the experimental and predicted ECD spectra. The bicyclo[4.1.0]heptane nucleus in compounds 1 and 2 was rarely found in nature. The known compounds were identified to be sesquicaranoic acid B (2), 9-cycloneren-3,7,11-triol (3), fumiquinazoline J (4), versiquinazoline H (5), penipurdin A (6), questinol (7), 2-hydroxy-4-ethoxybenzoic acid (8). Compounds 4 and 7 exhibited moderate cytotoxicity against MCF-7 and K562 tumor cell lines, with IC50 values ranging from 12.6 to 37.1 M.
DOI
http://doi.org/10.25135/rnp.453.2402.3149
Keywords
marine fungus
Penicillium
sesquiterpene
penicipeneacid
Available online: April 08, 2024
DETAILS
DOWNLOAD PDF
© ACG Publications. All rights reserved.
An investigation into the secondary metabolites of the sea sediment-derived Penicillium sp. LPFH-ZW-1 led to the isolation of a new compound, named penicipeneacid (1), along with seven known compounds. The structures of these compounds were identified through extensive analysis of the 1D and 2D NMR data, combined with HRESIMS data. The absolute configuration of 1 was determined by comparing the experimental and predicted ECD spectra. The bicyclo[4.1.0]heptane nucleus in compounds 1 and 2 was rarely found in nature. The known compounds were identified to be sesquicaranoic acid B (2), 9-cycloneren-3,7,11-triol (3), fumiquinazoline J (4), versiquinazoline H (5), penipurdin A (6), questinol (7), 2-hydroxy-4-ethoxybenzoic acid (8). Compounds 4 and 7 exhibited moderate cytotoxicity against MCF-7 and K562 tumor cell lines, with IC50 values ranging from 12.6 to 37.1 M.
DOI
http://doi.org/10.25135/rnp.453.2402.3149
Keywords
marine fungus
Penicillium
sesquiterpene
penicipeneacid
Available online: April 08, 2024
DETAILS
DOWNLOAD PDF
© ACG Publications. All rights reserved.
Antimicrobial and α-Glucosidase Inhibitory Compounds from the Branches of Uvaria siamensis
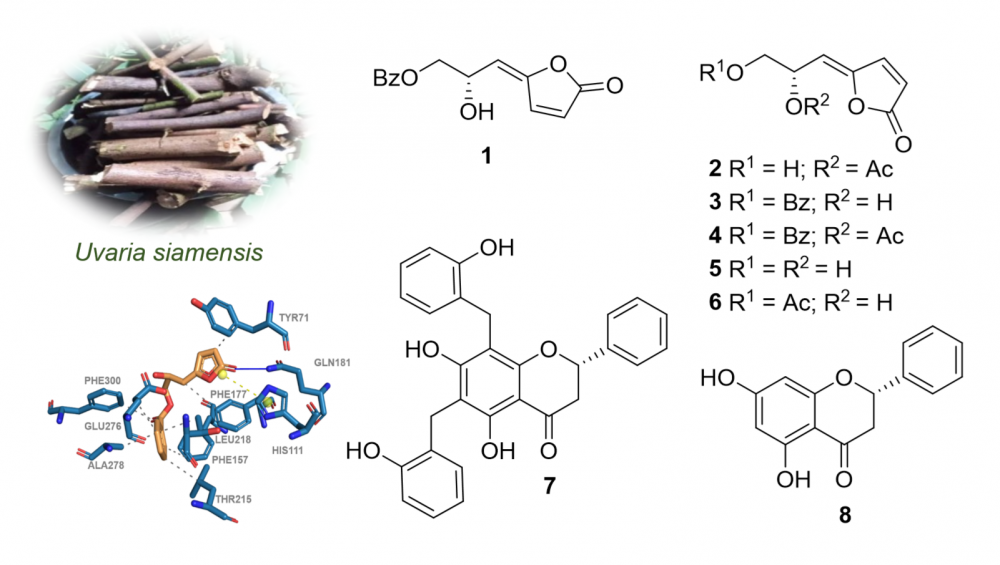
A new γ-butenoid, (Z)-6-acetylsphaerodol (2), along with seven known compounds, (4E)-7-benzoyloxy-6-hydroxy-2,4-heptadien-4-olide (1), (6S)-melodorinol (3), (6S)-acetylmelodorinol (4), (Z)-sphaerodiol (5), (Z)-7-acetylsphaerodol (6), dichamanetin (7), and pinocembrin (8), were isolated and structurally elucidated from Uvaria siamensis growing in Thailand. Their chemical structures were assigned by extensive spectroscopy (NMR and HRESIMS), as well as comparisons with the previous literature. Compounds 1-8 were evaluated for the antimicrobial activity against two multidrug-resistant Staphylococcus aureus strains, 23Sa1 and SA-ATCC. Compounds 4 and 6 displayed moderate activity with inhibitory zones of 13 and 12 mm against 23Sa1 and SA-ATCC strains, respectively. Compounds 3 and 7 exhibited significant efficacy, displaying IC50 values of 157.7 and 94.2 µM, respectively, against α-glucosidase. According to the docking data, compounds 3 and 7 have a greater influence on binding contacts with the α-glucosidase active pocket, thereby affecting the inhibitory activity of the enzyme.
DOI http://doi.org/10.25135/rnp.452.2402.3145 Keywords Annonaceae Uvaria siamensis (Z)-6-acetylsphaerodol γ-butenoid antibacterial activity α-glucosidase inhibition Available online: April 08, 2024 DETAILS DOWNLOAD PDF © ACG Publications. All rights reserved.A Novel Limonoid from the Seeds of Chisocheton macrophyllus
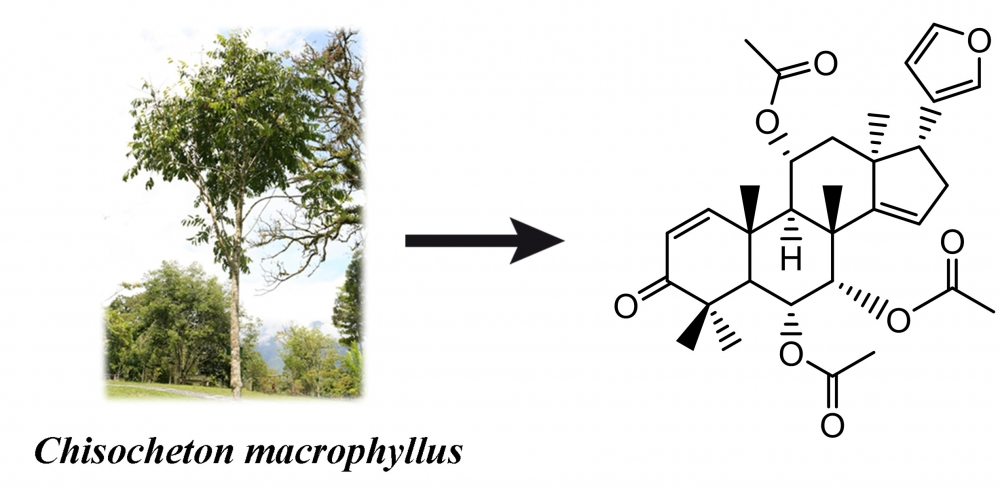
This study aimed to isolate a novel limonoid compound, (6R,7S, 8R, 9R, 10R, 11R, 13S, 17R)-17-(furan-3-yl) -4, 4, 8, 10, 13-pentamethyl-3-oxo-4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 16, 17-dodecahydro-3H-cyclopenta[a]phenanthrene-6,7,11-triyltriacetate (11α-acetoxydysobinin) (1), and 3 pre-existing limonoids, namely dysobinin (2), dysobinol (3), and 7-deacetylepoxyazadiradione (4) from the seeds of Chisocheton macrophyllus (Meliaceae). In addition, the structure of the isolated compounds was determined using various spectroscopic techniques, including UV, IR, HRTOFMS, 1D, and 2D NMR. The cytotoxic effects of each compound were then evaluated against breast cancer cells of the Michigan Cancer Foundation-7 (MCF-7), but no significant activity was observed.
DOI http://doi.org/10.25135/rnp.451.2311.2960 Keywords Chisocheton macrophyllus Meliaceae limonoid cytotoxic activity MCF-7 Available online: March 17, 2024 DETAILS DOWNLOAD PDF © ACG Publications. All rights reserved.Chemical Constituents and Pharmacological Activities of the Genus Nageia
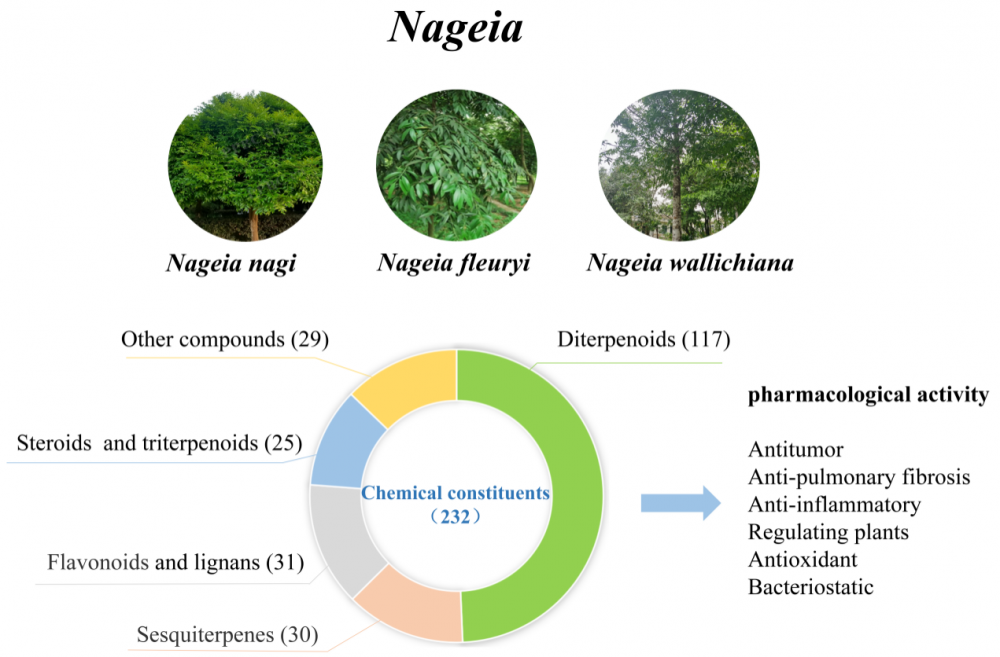
There are seven species in the genus Nageia, a member of the Podocarpaceae family; five are found in China. They are primarily found in eastern and southern Asia, close to the equator at the 30th parallel north and south, and in coastal mountainous areas and islands in the western Pacific. These herbs are extremely valuable for medicinal purposes. The three species of Nageia that are the subject of most current research on their chemical composition and pharmacological properties are N. nagi, N. fleuryi, and N. wallichiana. There are currently 232 known chemical components, which include steroids, flavonoids, sesquiterpenoids, and diterpenoids. Of these, 86 diterpenoid dilactones comprise most of the chemical components, and only plants of the Nageia genus contain C-ring decarbonized diterpenoid dilactones. These plants exhibit promising pharmacological activities such as anti-tumor, antioxidant, antibacterial, and anti-inflammatory properties. For the first time, an in-depth and systematic evaluation of the chemical constituents and pharmacological properties of plants in the Nageia genus is presented in the this article. This theoretical framework will help in the future investigation and development of medicinal resources and active ingredients within the genus.
DOI http://doi.org/10.25135/rnp.435.2311.2979 Keywords Nageia diterpenoids nagilactones antitumor antioxidants bacteriostatic Available online: February 21, 2024 DETAILS DOWNLOAD PDF © ACG Publications. All rights reserved.
