JOURNAL 3035
Organic Communications
Year: 2024 Issue: 1 January-March
p.38 - 55
Viewed 2076 times.
GRAPHICAL ABSTRACT
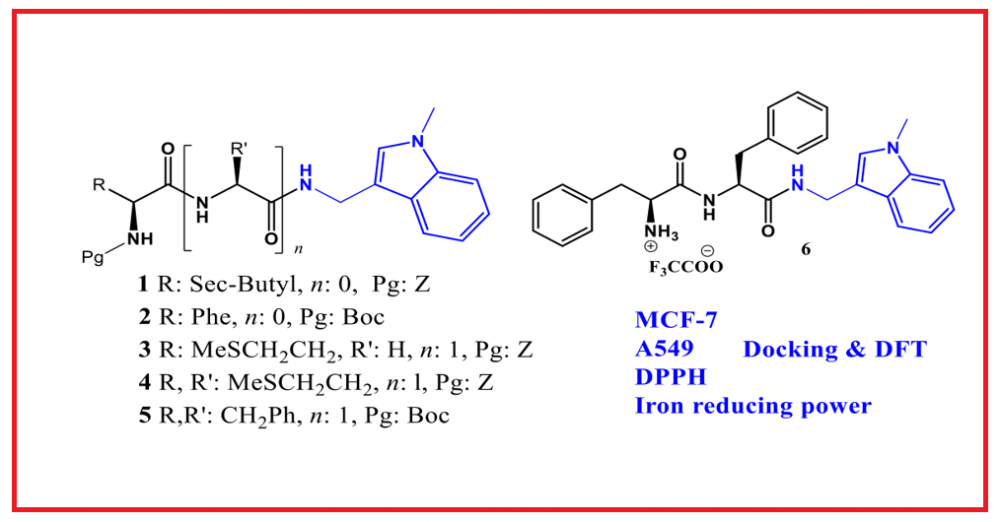
ABSTRACT
:In this study, the structures of six new peptide-indole derivatives were elucidated through spectroscopic and analytical methods following their synthesis. In addition to their anticancer and antioxidant properties, density functional theory (DFT) calculations and docking studies were conducted for the compounds. According to the obtained results, compounds 1 and 3 were identified as the most active against the MCF-7 cell line, with IC50 values of 8.72 and 5.86 μg/mL, respectively. Conversely, compounds 4 and 1 were found to be the most active against the A549 cell line, with IC50 values of 15.43 and 16.10 μg/mL, respectively. When compared to standard antioxidants using both the DPPH and iron reduction power assays, the compounds did not exhibit significant antioxidant activity. The molecular geometry and electronic properties of the synthesized peptide-indole derivatives were investigated through theoretical calculations using the Density Functional Theory (DFT) method. Molecular docking studies were also conducted to investigate the binding modes of the synthesized compounds within the active sites of EGFR enzyme.
KEYWORDS- Indole derivatives
- peptides
- anticancer
- antioxidant
- DFT calculations
- docking study