JOURNAL 3124
Organic Communications
VOLUME & ISSUE
Year: 2024 Issue: 1 January-March
Year: 2024 Issue: 1 January-March
PAGES
p.56 - 62
p.56 - 62
STATISTICS
Viewed 2232 times.
Viewed 2232 times.
GRAPHICAL ABSTRACT
ABSTRACT
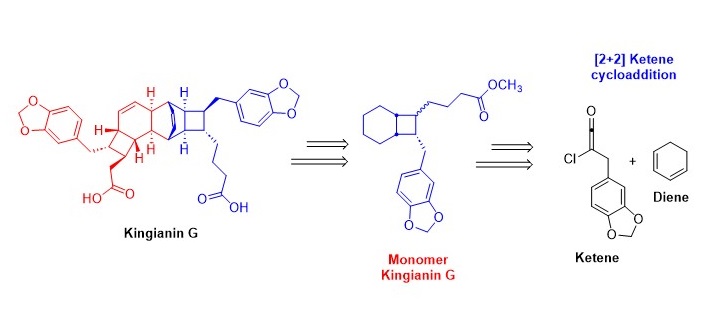
Kingianins are pentacyclic polyketides isolated from Endiandra kingiana. They exhibit promising activity in inhibiting anti-apoptotic proteins (i.e Bcl-xL and Mcl-1) and anti-diabetic proteins (α-glucosidase) at micromolar levels. Due to their structural complexity and intriguing biological activity, researchers are increasingly focussing on designing total synthesis routes for these compounds. In this communication, we propose a new non-biomimetic synthesis strategy that utilises a [2+2] ketene cycloaddition reaction as a key-step to access the bicyclo[4.2.0]octane system of kingianins. To the best of our knowledge, this is the first report based on this approach.
KEYWORDS- Kingianins
- cyclic polyketides
- bicyclo[4.2.0]octane system
- [2+2] ketene cycloaddition reaction