Records of Natural Products
Year: 2025 Volume: 19 Issue:3 May-June
1) Malaysian Herbaceous and Shrub Species with Anti-Obesity Properties: A Review
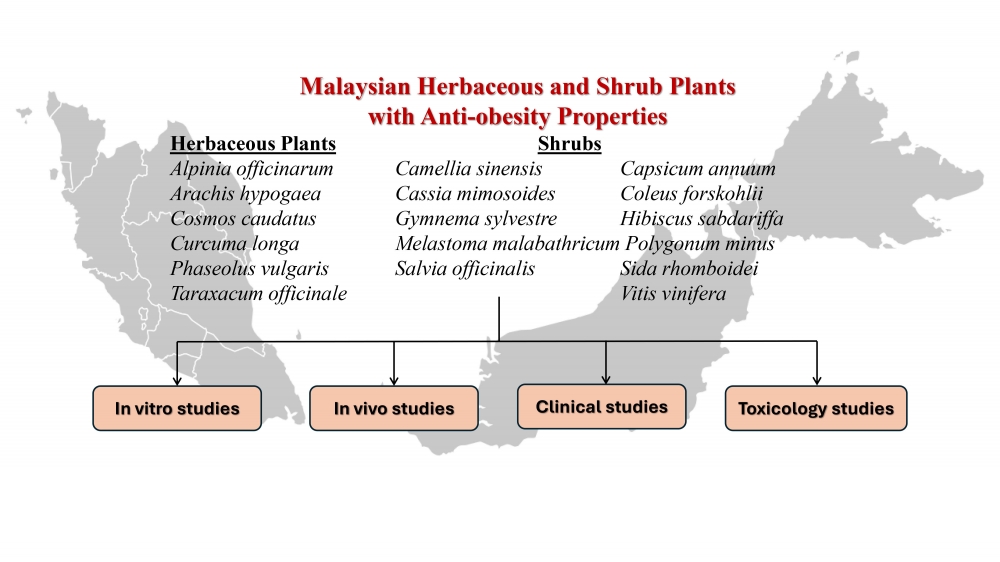
Obesity is a widespread public health problem worldwide and is associated with numerous health risks, requiring effective prevention and treatment strategies. Although synthetic anti-obesity drugs are available, they often cause adverse side effects, thus generating interest in natural products as safer and more effective alternatives. Malaysian shrub plants thrive in the country’s tropical climate and offer a rich source of biological active compounds with potential anti-obesity effects. Several studies, including in vivo and clinical trials, have reported promising anti-obesity effects of these plants. Research on local plants not only aligns with public health priorities but also provides affordable interventions for the local population. This review systematically summarizes findings from 193 articles across electronic databases using relevant keywords, covering Malaysian herbaceous and shrub species with anti-obesity potential, their isolated compounds, proposed mechanisms of action, and supporting in vitro, animal model, and clinical evidence. In addition, toxicological data are discussed to provide a comprehensive perspective of the therapeutic potential of these plants. This review highlights the gaps in current knowledge and proposes future directions for leveraging Malaysian plants to combat obesity.
DOI http://doi.org/10.25135/rnp.512.2412.3385 Keywords Obesity anti-obesity herbaceous plants shrubs DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.2) Two New Diketopiperazine Alkaloids from a Deep-soil Derived Fungus Penicillium simplicissimum GZWMJZ-1612
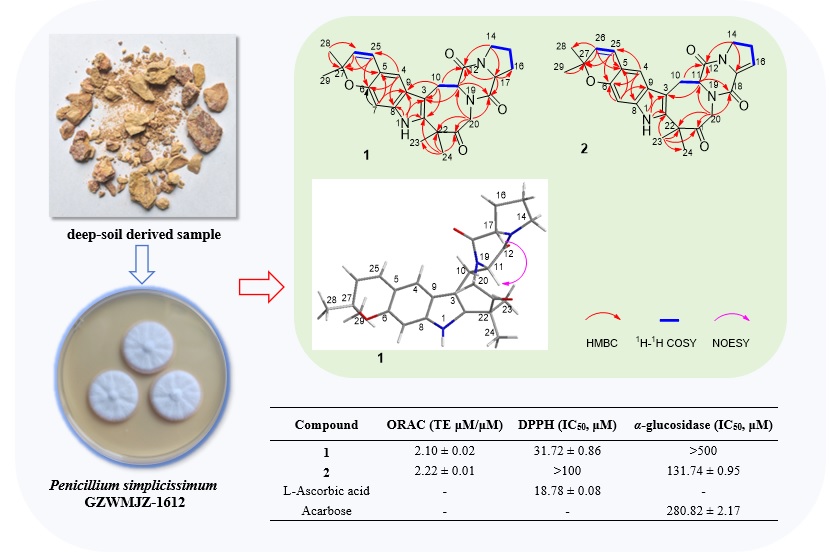
Two new diketopiperazine alkaloids, namely simplicamides A (1) and B (2), were isolated from the deep-soil derived fungus Penicillium simplicissimum GZWMJZ-1612. The structures of these two compounds were determined by NMR, HRESIMS, ECD calculation, and Marfey’s method. Compound 1 exhibited potent antioxidant capacity of ORAC and DPPH scavenging ability. Compound 2 displayed significant ORAC scavenging ability and α-glucosidase inhibitory activity.
DOI http://doi.org/10.25135/rnp.510.2502.3430 Keywords deep-soil Penicillium simplicissimum diketopiperazine alkaloids antioxidant capacity α-glucosidase DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.3) Enzyme Inhibition Properties of Calendula officinalis, Matricaria chamomilla, and Anthemis pseudocotula: Kinetics and Molecular Docking Studies
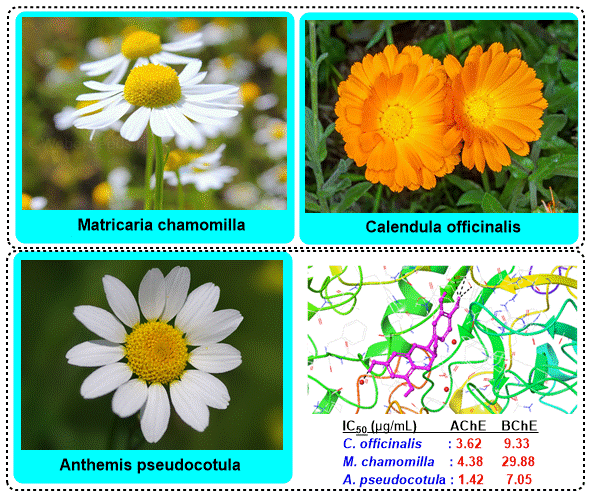
This study determined the enzyme inhibition potential of three species (Calendula officinalis, Matricaria chamomilla, and Anthemis pseudocotula) from the Asteraceae family through in silico, followed by in vitro studies. Quinic acid, fumaric acid, gallic acid, chlorogenic acid, vanillic acid, quercetin, apigenin, and isorhamnetin were determined by LC-MS/MS in all of the species. Metabolic enzymes are essential catalysts regulating biochemical reactions within living organisms, facilitating energy production, detoxification, and biosynthesis. These enzymes play a crucial role in maintaining cellular homeostasis and are tightly regulated to ensure optimal metabolic function. High docking scores were also obtained for butyrylcholinesterase (BChE), α-glycosidase, α-amylase, and human carbonic anhydrase I and II enzymes (hCA I and hCA II). Among the extracts, Anthemis pseudocotula was concluded to be the best inhibitor for the enzymes, which was further determined by in vitro enzyme inhibition tests. Besides, it was concluded that all extracts showed anti-cholinergic, anti-diabetic, and anti-glaucoma properties. This is the first study determining the enzyme inhibition property of Anthemis pseudocotula and the three species' hCA I and hCA II inhibition activities.
DOI http://doi.org/10.25135/rnp.1505.2412.3383 Keywords Enzyme inhibition Matricaria chamomilla Anthemis pseudocotula Calendula officinalis LC-MS/MS DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.4) Chemical Composition, Antioxidant, Acetylcholinesterase and β-Lactamase Inhibitory Activities of Essential Oils from Clerodendrum cyrtophyllum Turcz. and Clerodendrum fortunatum L.
(1).png)
Clerodendrum cyrtophyllum Turcz. and Clerodendrum fortunatum L. are traditional medicinal plants used for clearing heat, reducing fire, reducing inflammation and detoxification, and relieving cough and analgesia in China. In the current study, the essential oils (EOs) of C. cyrtophyllum and C. fortunatum were obtained by hydrodistillation. The chemical compositions and the contents of the EOs were measured by GC-MS and GC-FID methods. The antioxidant capacity was measured by 1,1-diphenyl-2-picryl-hydrazyl radical (DPPH), 2'-azinobis(3-ethylbenzthiazoline-6-sulphonate) (ABTS) free radical scavenging capacity and ferric reducing antioxidant power (FRAP). The GC and GC-MS analysis results identified 89 and 63 components in the EOs of C. cyrtophyllum and C. fortunatum, accounting for 94.9% and 96.2%, respectively. 1-octen-3-ol, linalool, and hexahydrofarnesyl acetone were reported as the same primary components. The inhibition rates of the DPPH radical scavenger assay were 43.49% ± 0.95% and 27.23% ± 0.40% at 20 mg/mL, while the ABTS radical scavenging assay showed capacities with the IC50 values 2.31 ± 0.05 mg/mL and 7.43 ± 1.80 mg/mL, respectively. The FRAP values (Trolox equivalent antioxidant concentration) were 55.61 ± 2.56 µmol/g and 23.39 ± 1.58 µmol/g, respectively. The C. cyrtophyllum and C. fortunatum EOs showed anti-acetylcholinesterase activity with the IC50 values 289.10 ± 0.43 µg/mL and 1060.00 ± 0.82 µg/mL, and β-lactamase inhibitory activity with the IC50 values 41.34 ± 0.84 µg/mL and 673.50 ± 1.27 μg/mL, respectively. This study makes a substantial contribution to the chemical and biological knowledge expansion on Clerodendrum species EOs from China.
DOI http://doi.org/10.25135/rnp.518.2501.3418 Keywords Clerodendrum cyrtophyllum Turcz. Clerodendrum fortunatum L. essential Oil GC-MS cemical compositions biological activities DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.5) Antibacterial Evaluation of Matricaria recutita L., Achillea millefolium L. Essential Oil and Tetracycline Combinations in Respect to in vivo Toxicity Data¥
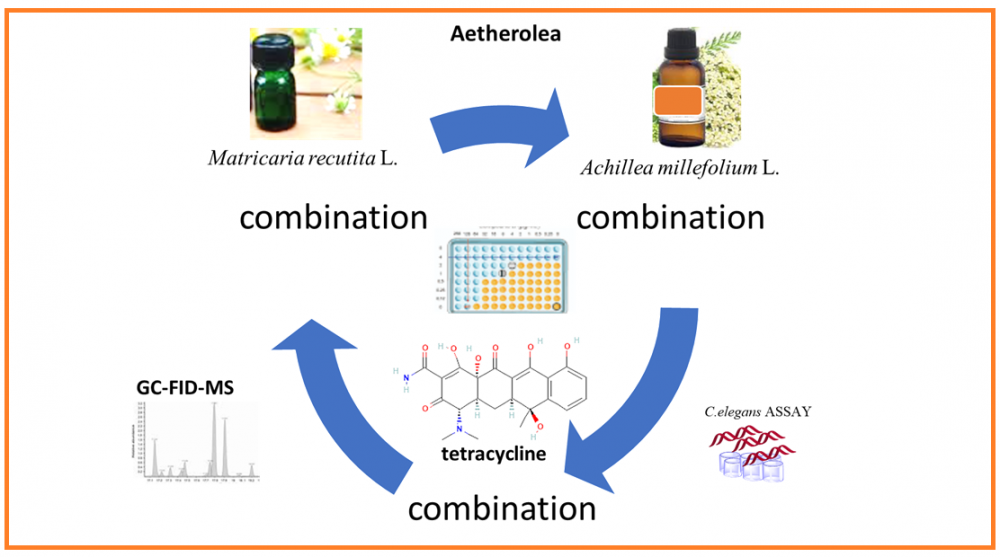
Matricaria recutita L. and Achillea millefolium L. (Astereacea) aetherolea are among the herbal drug preparations used due to their broad-spectrum antimicrobial effects. The present study aimed to evaluate the in vitro antibacterial activity of M. recutita and A. millefolium oils individually combined with tetracycline. Followed by safety/toxicity evaluation using an in vivo animal alternative experimental model, namely Caenorhabditis elegans. Chemical verification of Pharmacopoeia quality essential oils was performed both by GC-FID and GC-MS systems, simultaneously. β-Caryophyllene (17%), β-pinene (13.2%), camphor (10%), and sabinene (9.7%) were identified as major components for A. millefolium essential oil; whereas, bisabolol oxide A (41.6%), α-bisabolol (19.4%), (E)-β-farnesene (17%), α-bisabolol oxide B (5.2%), α-bisabolon oxide A (5%), chamazulene (1.6%), and germacrene D (1.2%) were determined as major components for M. recutita essential oil, respectively, in line with the international standards. Antibacterial activities of essential oils and tetracycline were evaluated by microdilution methods against the standard pathogenic strains Bacillus cereus NRRL B3711, Corynebacterium striatum ATCC BAA-1293, Streptococcus sanguinis ATCC 10556, Staphylococcus aureus ATCC 700699. Minimal inhibitory concentrations (MIC) were determined followed by the checkerboard combination studies. A. millefolium and M. recutita essential oils showed in vitro inhibitory activity against all tested microorganisms (MIC= 48.7-6250 μg/mL). The oil combinations with tetracycline showed varying inhibitory antibacterial activity, where M. recutita essential oil with tetracycline resulted in synergism against S.aureus. In vivo toxicity tests on C. elegans nematodes resulted in a non-acute toxicity, indicating the relatively safe use of the tetracycline combinations.
DOI http://doi.org/10.25135/rnp.517.2503.3455 Keywords Matricaria recutita L Achillea millefolium L. Caenorhabditis elegans synergy antimicrobial activity. DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.6) A New Lignan from the Leaves and Stems of Melaleuca bracteata F. Muell.
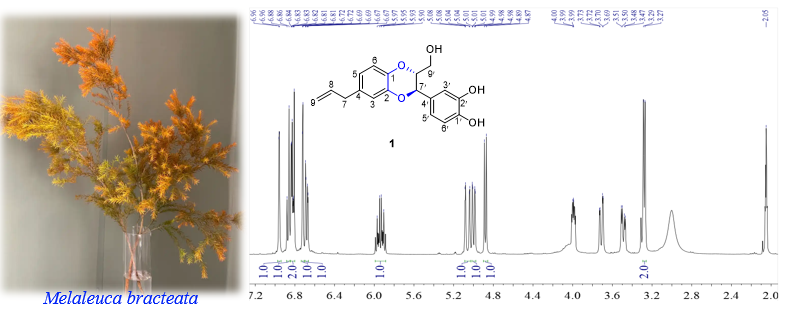
Lignans, such as podophyllotoxin, schisandrin, and silymarin, are known for their notable biological activities. In our study, a new lignan, named melaleucin D (1), was isolated from the leaves and stems of the plant Melaleuca bracteata F. Muell. The planar structure was determined by combining 1H NMR/13C NMR/ COSY/HSQC/HMBC spectra and HRESIMS data. The absolute configuration was established by comparing the experimental circular dichroism (CD) data with the calculated theoretical ECD data. Compound 1 contained an unusual dioxane moiety. The antibacterial assay revealed that compound 1 did not exhibit a zone of inhibition against Staphylococcus aureus ATCC 6538 and Escherichia coli ATCC 25922 at a concentration of 256 mg/mL.
DOI http://doi.org/10.25135/rnp.503.2502.3427 Keywords Melaleuca bracteata F. Muell. lignan separation structure elucidation DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.7) A New Clerodane-type Diterpene from Conyza blinii Levl.
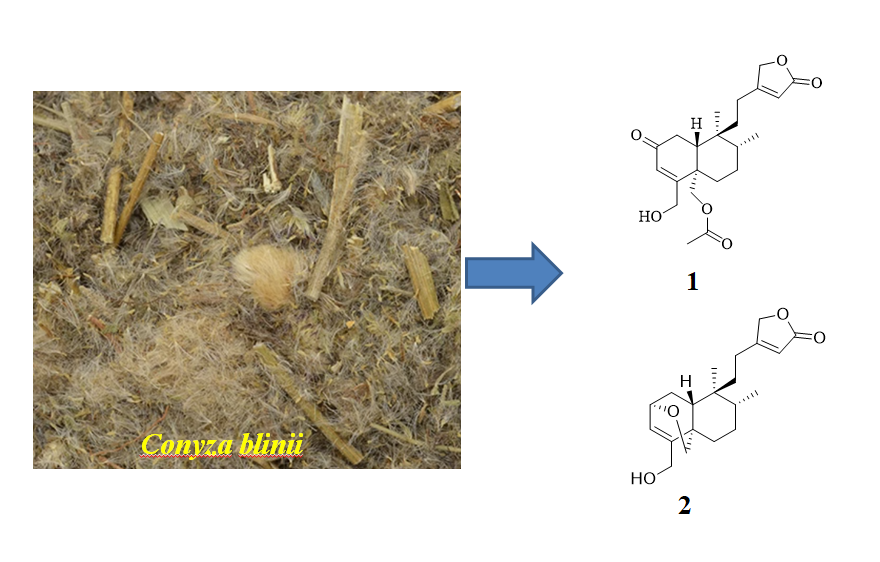
A new clerodane-type diterpene, conbliate C (1), along with three known analogues (2−4), were isolated from Conyza blinii Levl. The structure of the new compound was elucidated on the basis of detailed spectroscopic analysis. The cytotoxic effects of the isolated compounds on two human tumors cell lines (AsPC-1 and HepG-2) were evaluated by the MTT assay.
DOI http://doi.org/10.25135/rnp.511.2502.3442 Keywords Asteraceae Conyza blinii Clerodane-type diterpenoid cytotoxicity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.8) Structures and Biological Evaluation of 8,4′-oxyneolignans from the Roots of Platycodon grandifloras
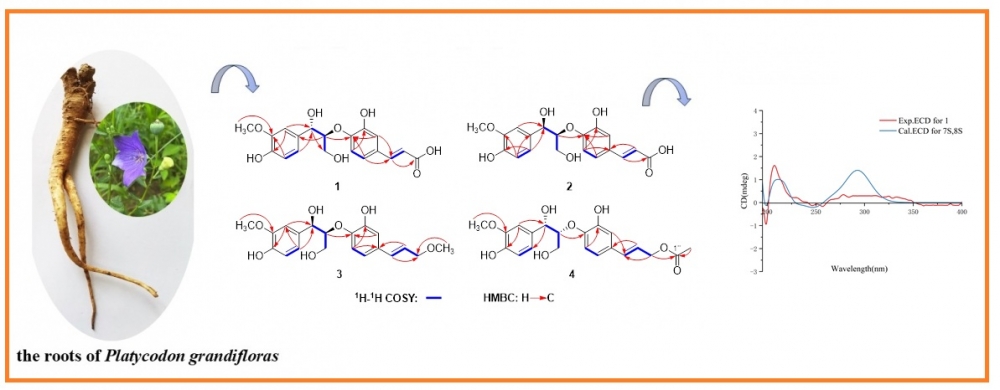
Chemical investigations of Platycodon grandiflorus have resulted in the isolation and identification of seven 8,4'-oxyneolignans, including four undescribed compounds (1-4). The structures of these novel compounds were determined using HR-ESI-MS and NMR (1D and 2D) spectroscopic analyses, combined with ECD calculations. The inhibitory activity of these terpenoids against α-glucosidase was also evaluated, and the results revealed that none of the compounds exhibited significant α-glucosidase inhibitory activity at a concentration of 50 μM. Compared with the positive control, all the compounds displayed weak inhibitory activity, with inhibition rates ranging from 1.06% to 7.31%.
DOI http://doi.org/10.25135/rnp.513.2501.3409 Keywords Platycodon Platycodon grandiflorus apha-glucosidase chemical constituents 8,4-oxyneolignan. DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.9) Fusacintone A, a New Polyketide from the Endophytic Fungus Fusarium tricinctum from Fritillaria monantha
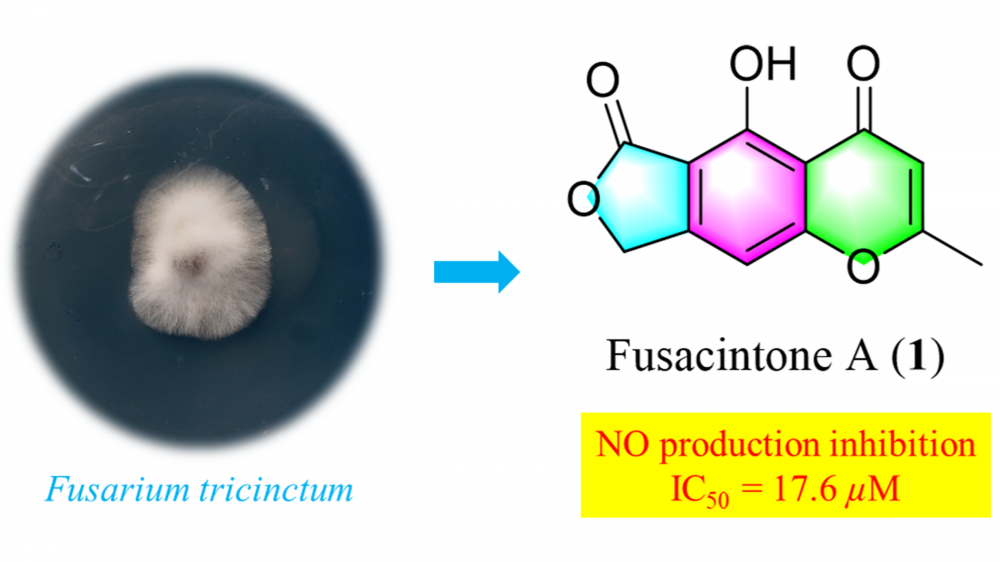
A previously undescribed polyketide (1) and four known compounds (2-5) isolated from the endophytic fungus Fusarium tricinctum from Fritillaria monantha. Compound 1 showed anti-inflammatory activity by inhibiting NO production with an IC50 value of 17.6±0.46 µM.
DOI http://doi.org/10.25135/rnp.514.2503.3464 Keywords Fusarium tricinctum endophytic fungus polyketide structural elucidation anti-inflammatory DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.