Records of Natural Products
Year: 2025 Volume: 19 Issue:5 September-October
1) Natural Polyphenols Against Cerebral Ischemia and Mechanism of Action: A Review
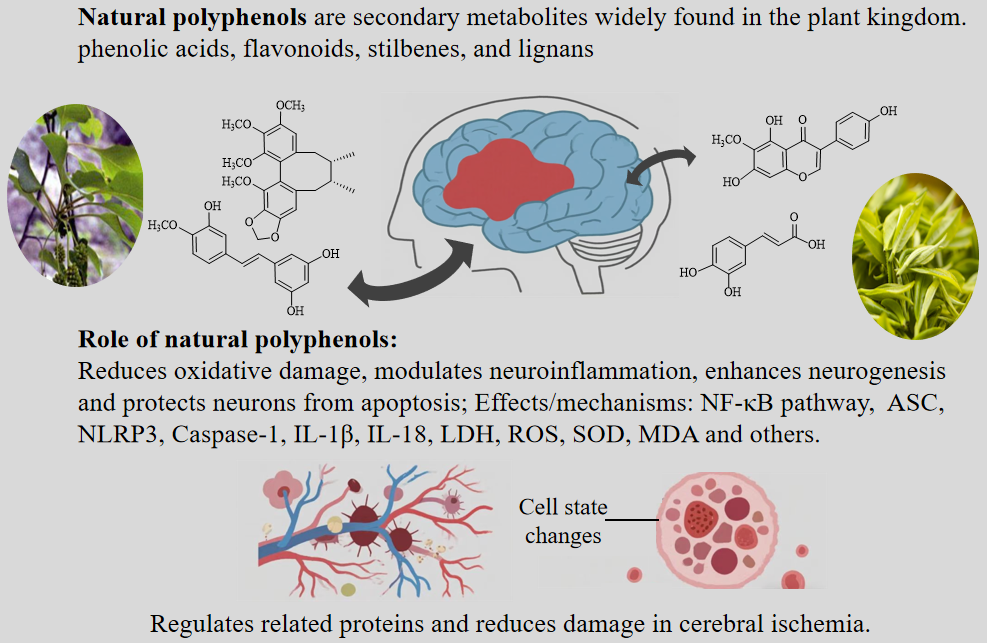
Cerebral ischemia is a disorder characterized by insufficient blood flow to the brain, which can lead to severe neurological deficits and brain damage. Given the complexity of cerebral ischemia and the limitations of current pharmacological treatments, there is a growing scholarly interest in natural polyphenols. Polyphenols are a diverse group of secondary metabolites, and these compounds have a strong antioxidant capacity, and oxidative stress is one of the main causes of post-ischemic brain damage. A major consequence of ischemia is the interruption of normal cellular processes due to oxygen and glucose deprivation, resulting in the production of reactive oxygen species (ROS). These ROS damage cellular structures, trigger an inflammatory response, and exacerbate neuronal death. In addition, some polyphenols have a direct effect on the cerebral vasculature; they improve endothelial function and enhance microcirculation, which is essential for restoring blood flow and oxygenation to ischemic brain tissue. Polyphenols can also affect mitochondrial function by promoting mitochondrial biogenesis and reducing mitochondrial dysfunction, which plays a key role in cell death during ischemic episodes. Therefore, this paper reviews the potential therapeutic effects of natural polyphenols on cerebral ischemia, focusing on their anti-ischemic effects and related mechanisms.
DOI http://doi.org/10.25135/rnp.543.2504.3507 Keywords Natural polyphenols antiischemic phytochemistry pharmacology DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.2) Promising Natural Polyphenols from Olive (Olea europaea) Leaves and Seeds: Dual Benefits in the Prevention of Ultraviolet B-induced Fibroblast Skin Damage and Anti Skin Hyperpigmentation
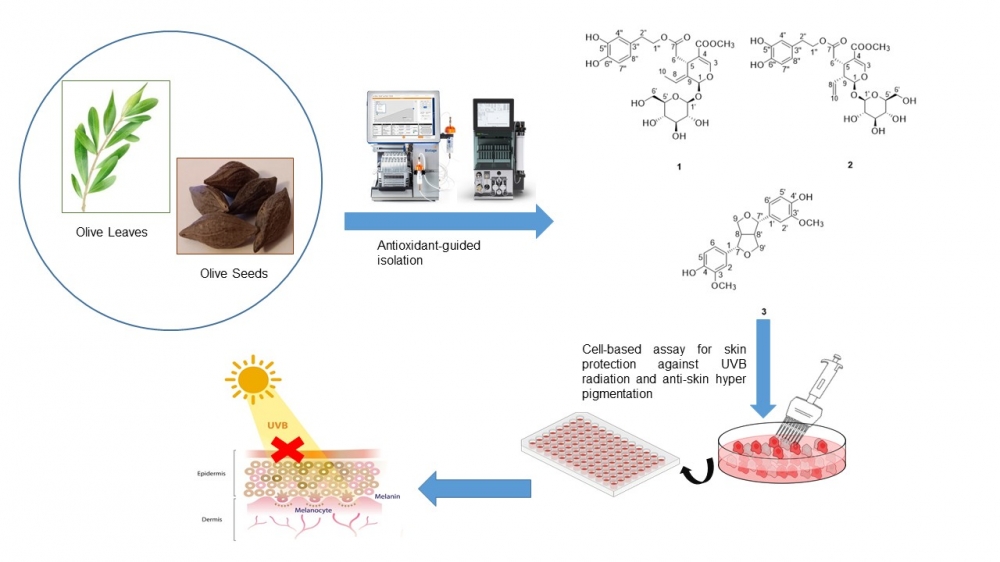
The development of phytochemicals from olive leaves and seeds for cosmeceutical applications is still scarce. This study aimed to perform comprehensive investigations of the bioactive compounds and biological activities of olive leaves and seeds for antioxidant, skin protection from ultraviolet B (UVB) irradiation in human dermal fibroblast (NHDF) cells from adult donors, and anti-melanogenesis in B16 melanoma cells. Two bioactive compounds were isolated from olive leaves, identified as oleuropein (1) and oleuroside (2). Additionally, one bioactive compound, pinoresinol (3) was elucidated for the first time from olive seeds. Among these compounds, pinoresinol demonstrated the most potent melanin inhibitory in B16 melanoma cells at the low concentration of 1.25 μg/mL with no cytotoxicity, decreasing the melanin content to 54 ± 5.48 %. In addition, oleuropein at 100 μg/mL inhibited melanin production to 35 ± 2.15 %, without cytotoxicity. Furthermore, oleuropein at concentrations of 100-200 μg/mL promoted cell proliferation above 120 % and exhibited skin protective activity at 200 μg/mL against UVB irradiation (250 mJ/cm2), maintaining cell viability to 76.54 ± 5.05 %. This study highlights the isolated compounds from olive leaves and seeds, which are agricultural by-products, offer potential natural sunscreen products in the future with fewer side effects.
DOI http://doi.org/10.25135/rnp.544.2506.3557 Keywords Natural polyphenols cytotoxic assay skin protectant anti melanogenesis cosmetic DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.
3) A New Acyclic Compound from the Medicinal Plant Tinospora sinensis

In this study, chemical investigation of the aerial parts of the medicinal plant Tinospora sinensis yielded five compounds (1-5), including a new acyclic compound (1) and four known compounds (2-5). Their structures were determined by extensive analysis of the spectroscopic data, including 1D (1H and 13C NMR) and 2D NMR data (HSQC, COSY, and HMBC). Compounds 3-5 were identified to be stearic acid (3), abscisic acid (4), and blumenol A (5) based on comparisons of the NMR data with those reported in the literature. The NMR data for 2-hydroxy methyl stearate (2) were reported for the first time in CDCl3 in this study. The compounds were evaluated for their antitumor potential against A549 cell line, while all were inactive (25 mM). Besides, compound 1 demonstrated marginal inhibition (19.35%) of LPS-induced NO production in RAW 264.7 cells (50 mM).
DOI http://doi.org/10.25135/rnp.530.2505.3536 Keywords Tinospora sinensis acyclic compound NMR DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.4) Anti-inflammatory Diterpenoid Alkaloids from Aconitum taipeicum
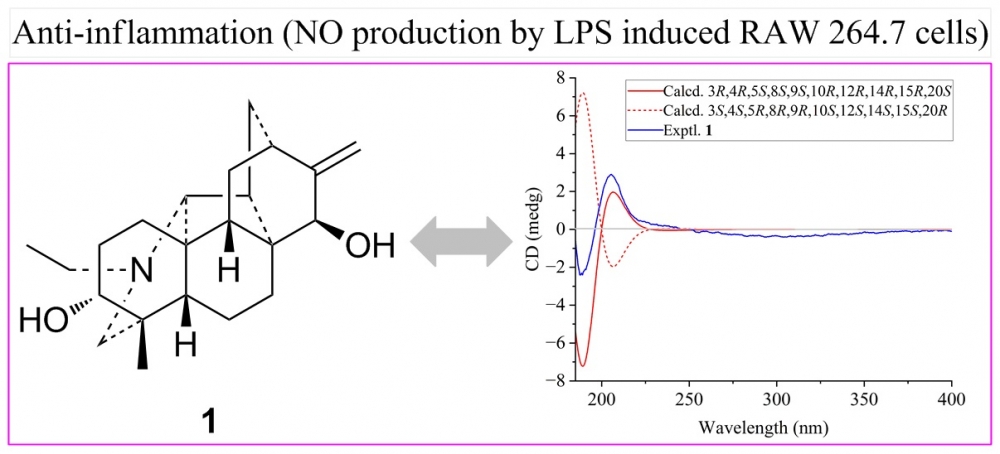
Aconitaimine A, a new hetidine-type C20-diterpenoid alkaloid (1), and three known aconitine-type C19-diterpenoid alkaloids (2-4) were isolated from the roots of Aconitum taipeicum. The structures of all isolates were identified by spectroscopic methods and comparison with literature data. Compounds 1 and 2 showed inhibition of nitric oxide production in RAW 264.7 macrophages stimulated by lipopolysaccharide with IC50 values of 30.5 and 4.7 μM compared to positive control (dexamethasone, IC50 = 12.9 μM).
DOI http://doi.org/10.25135/rnp.536.2506.3565 Keywords Aconitum taipeicum Ranunculaceae diterpenoid alkaloids NO inhibitory activity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.5) Cyclocarioside Z14, A New Dammarane Triterpenoid Glycoside from The Leaves of Cyclocarya paliurus with Cytotoxicity
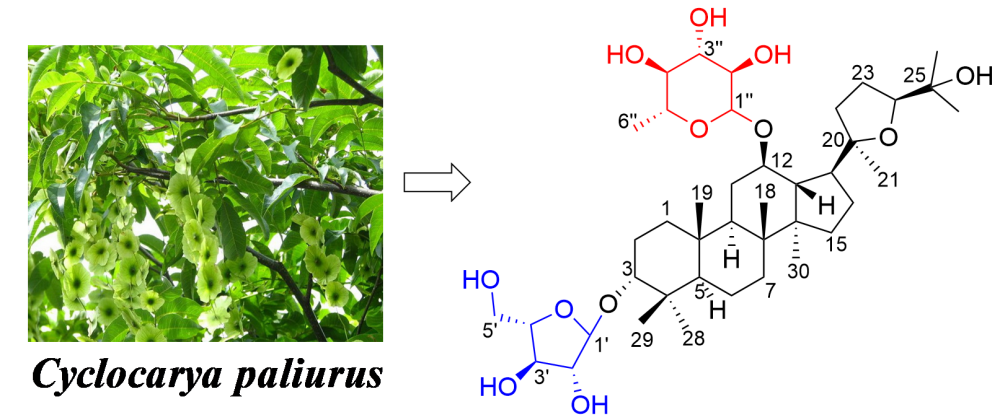
A new dammarane triterpenoid glycoside cyclocarioside Z14 (1) and four known compounds (2-5) were isolated from the dichloromethane extract of the leaves of Cyclocarya paliurus. The chemical structures were elucidated by the extensive spectroscopic data analysis of NMR, HR-ESI-MS, and acid hydrolysis. All isolated compounds were assayed on the cytotoxicity against seven human cancer cell lines. The compounds 1 and 3 showed moderate cytotoxicity against MCF-7 cells with an IC50 value of 29.51 μM and 33.88 μM.
DOI http://doi.org/10.25135/rnp.537.2505.3525 Keywords Cyclocarya paliurus dammarane triterpenoid glycoside structural elucidation cytotoxicity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.6) An Undescribed Zhepiresionol Analogue from Ailanthus altissima
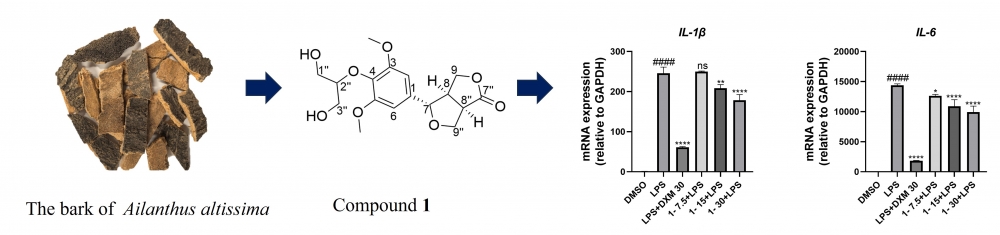
This study involved the extraction, isolation, structural elucidation of a new analogue of zhepiresionol, as well as five lignans previously identified, from the plant Ailanthus altissima (Mill.) Swingle. The structural elucidation was accomplished through comprehensive analyses of various spectroscopic data, which included HRESIMS, UV, IR, and NMR, along with a comparison of ECD curves. At a concentration of 15 µM, Compound 1 showed the capacity to suppress IL-1β and IL-6 expression in RAW 264.7 cells that were Lipopolysaccharide-stimulated.
DOI http://doi.org/10.25135/rnp.538.2505.3520 Keywords Ailanthus altissima zhepiresionol analogue anti-inflammatory activity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.7) A New Polyketide Derivative from the Nicotiana tabacum Symbiotic Fungus Aspergillus japonicus TE-739D
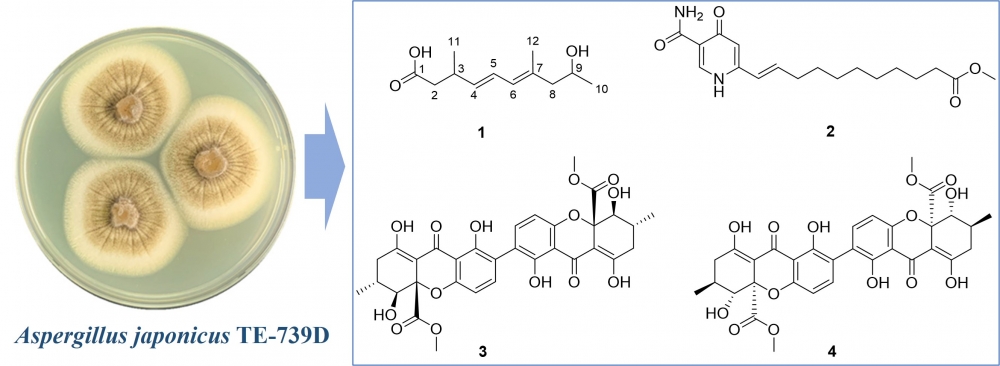
biological effects. In our study chemical exploration of the Nicotiana tabacum symbiotic fungus Aspergillus japonicus TE-739D led to the discovery of a new polyketide derivative, namely (3S,4E,6E,9S)-9-hydroxy-3,7-dimethyldeca-4,6-dienoic acid (1), along with three previously reported compounds 2−4. The structures of these compounds were elucidated by using HRESIMS, NMR spectroscopic analyses, and quantum chemical calculations. Compounds 3 and 4 showed strong antibacterial activity against four representative bacterial strains including two Gram-negative species (Agrobacterium tumefaciens and Xanthomonas oryzae) and two Gram-positive Bacillus species (B. cereus and B. subtilis), with MIC values range from 1 to 16 μg/mL, respectively.
DOI http://doi.org/10.25135/rnp.541.2506.3561 Keywords Secondary metabolites Aspergillus japonicus polyketides antibacterial activity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.8) A Novel Monoterpenoid Derivative Isolated from Chloranthus serratus Roots with Anti-inflammatory Activity
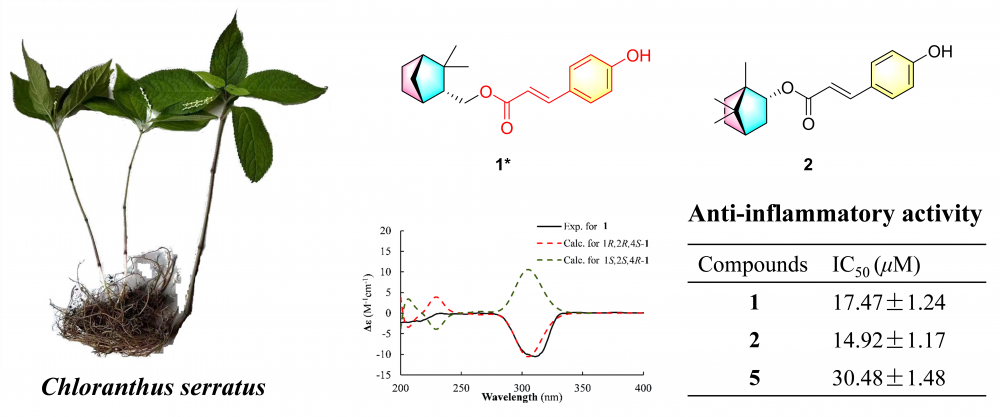
Eight compounds were isolated from the 95% ethanol extract of Chloranthus serratus roots, including two monoterpenoid derivatives (1, 2), one pimarane-type diterpenoid (3), and five labdane-type diterpenoids (4-8). Compound 1 was identified as a previously undescribed camphene derivative, while compound 3 was a new natural product. Their structures and relative configurations were elucidated using HR-MS, NMR and ECD calculations. Compounds 1, 2, 5 significantly inhibited nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW 264.7 cells, with IC50 values of 17.47±1.24, 14.92±1.17, and 30.48±1.48 μM, respectively.
DOI http://doi.org/10.25135/rnp.542.2506.3558 Keywords Chloranthus serratus monoterpenoid anti-inflammatory activity DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.